
Gastric cancer patient with MET amplification treated with crizotinib achieves long-term survival: a case report
Highlight box
Key findings
• A case of gastric cancer (GC) patient with hepatocyte growth factor receptor (MET) amplification who was treated with crizotinib for three years and gained an additional high quality of life during treatment.
What is known and what is new?
• MET amplification, having a poor prognosis, accounts for about 4–6% of GC patients. Crizotinib shows encouraging results with 8.1 months overall survival (OS) in chemo-refractory MET amplification esophageal and gastric adenocarcinomas.
• This case acquired three years of response with crizotinib therapy. Subsequently, the patient benefited from trametinib screened from the mini patient-derived xenograft (miniPDX) mouse model.
What is the implication, and what should change now?
• A patient with MET amplification GC could acquire good benefits from crizotinib.
• miniPDX mouse model could provide a reference for the patient-developed resistance and tumor progression and select treatment options.
Introduction
Based on the Global Cancer Statistics in 2018, gastric cancer (GC) is one of the most common digestive tract tumors, ranking fifth in cancer incidence worldwide. Additionally, GC is the third most common cancer leading to cancer-related deaths (1). According to the data from 2020, approximately 1.1 million individuals diagnosed with GC each year and 770,000 died from it (2). In general, the overall survival (OS) time of progressive GC patients is generally less than 12 months, and the five-year survival rate is less than 10% (3,4). As a highly heterogeneous tumor, advanced GC patients have been mainly treated with systemic chemotherapy in the past, but the efficacy of chemotherapy was still not satisfactory. Immunotherapy has recently become a promising treatment method, but the clinical results related to immune checkpoint inhibitors (ICIs) show that the clinical efficacy of a single agent is minimal. Clinical trials are currently exploring combination therapies, mainly chemotherapy (5). In recent years, the emerging of targeted therapy improved the survival and prognosis of GC patients.
As a member of the receptor tyrosine kinases (RTKs), hepatocyte growth factor receptor (MET) plays an important role in regulating tumor proliferation, differentiation, metastasis and survival. MET gene abnormalities occur in solid tumors such as lung, liver, stomach, breast, brain and colorectal cancers (6). MET amplification, having a poor prognosis, accounts for about 4–6% of GC patients (7). Up to now, the National Comprehensive Cancer Network (NCCN) guidelines have no therapeutic agents are recommended for MET amplification in GC (8). It has been reported that crizotinib (9), savolitinib (10,11) and cabozantinib (12) have shown significant effects in the treatment of MET amplification in GC patients.
Crizotinib, a selective small-molecule oral inhibitor of the anaplastic lymphoma kinase (ALK), MET, and ROS proto-oncogene 1 (ROS) RTKs, is currently approved for patients with high levels of MET amplification in non-small cell lung cancer (NSCLC) (13). However, it is not approved by the Food and Drug Administration (FDA) and National Medical Products Administration (NMPA) for GC patients with MET amplification, and still in clinical trials. In a large screened cohort (n=570), 35 patients were found to be MET amplified and 9 were treated with crizotinib. Crizotinib shows encouraging results with 55.6% best overall response rate (ORR) was 55.6%, 3.2 months median progression-free survival (PFS), and 8.1 months OS in chemo-refractory MET amplification esophageal and gastric adenocarcinomas (14). Here, we report a rare case of GC patient with MET gene amplification who treated with crizotinib for three years and gained an additional high quality of life during treatment. We present this following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-118/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. For the mouse model, all procedures were performed under a project license (No. 2021-KY-0192) granted by ethics board of Henan Cancer Hospital, in compliance with institutional guidelines for the care and use of animals.
In January 2019, a 64-year-old Chinese male patient presented to our hospital with abdominal distention, then he was diagnosed as a medium to low-differentiated adenocarcinoma of the stomach (cTxN0M1) by gastroscopy. Immunohistochemistry (IHC) results of the patient’s primary tumor showed MLH1(−), MSH2(−), MSH6(−), PMS2(−), and HER2(−). Programmed cell death ligand 1 (PD-L1) combined positive score (CPS) in primary was also negative. Subsequent laparoscopic exploration revealed peritoneal seeding. Pathology of the abdominal wall nodules biopsy showed metastatic adenocarcinoma. The patient’s primary tissues were subjected to next-generation sequencing (NGS, Genecast Biotechnology, Wuxi, China), and the results showed the presence of MET amplification with a copy number (CN) of 7.54 (Table 1). Intraoperative paclitaxel 30 mg intraperitoneal infusion chemotherapy was performed. After six cycles of chemotherapy with S-1 (tegafur, gimeracil and oteracil porassium capsules, 60 mg) on days 1–14 and nab-paclitaxel (200 mg) on days 1, 8, the patient’s best outcome was evaluated as stable disease (SD), but the patient discontinued the therapy in May 2019 after significant myelosuppression and intolerable side effects such as malaise and nausea. Since there was no suitable drug to choose from, so the patient was given crizotinib therapy based on the amplified CN of MET gene (CN 7.54) on May 22, 2019.
Table 1
| Gene | c.HGVS | p.HGVS | Functional region | Allele frequency |
|---|---|---|---|---|
| MET | Amplification | – | – | CN =7.54 |
| ALOX12B | c.180C>A | p.D60E | EX2 | 37.05% |
| BLM | c.2400_2401insTCGTTTTGTTATTGATGAAGCACATTGTGTC | p.Q802Ffs*4 | EX12 | 6.5% |
| NF1 | c.1845G>T | p.K615N | EX16 | 1.23% |
| PLCG2 | c.679G>A | p.V227M | EX8 | 17.34% |
| TET2 | c.3708_3716del | p.P1237_S1239delPLS | EX6 | 0.75% |
| TP53 | c.809T>G | p.F270C | EX8 | 38.86% |
c.HGVS, description of coding DNA (c.) variants by human genome variation society (HGVS); p.HGVS, description of protein (p.) variants by HGVS; CN, copy number.
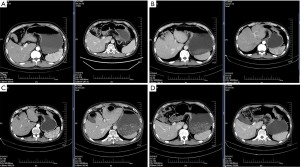
From 22 May 2019, the patient received crizotinib at a dose rate of 250 mg twice daily. At the beginning of treatment, chest computed tomography (CT) showed a slightly thickened localized stomach wall and a cyst in the left kidney (Figure 1A,1B). After one year of crizotinib treatment, the CT results showed a slight reduction in the thickness of the stomach wall (Figure 1C,1D). The above CT results indicated that the patient’s disease was stable during treatment with crizotinib. Thirty-four months later, the examination revealed a dramatic increase in tumor markers, such as carbohydrate antigen199 (CA199), which increased to 322.4 U/mL, about 16 times of the initial diagnosis. Meanwhile, positron emission tomography-CT revealed that tumor tissue was still metabolically active in the stomach, accompanied by pleural effusion, peritoneal metastasis and other symptoms. As of March 2022, the patient treated with crizotinib had a PFS of 34 months.
After disease progression (PD) occurred, due to lack of suitable treatments, the gastric tumor tissue was sequenced for a second time by target-capture sequencing of 1021-gene panel (Geneplus-Beijing Institute, Beijing, China). The sequencing results showed that MET amplification was no longer detected after crizotinib treatment. Other mutations, such as kirsten rat sarcoma viral oncogene homologue (KRAS), tumor protein p53 (TP53) mutations, caspase recruitment domain family member 11 (CARD11), lysine methyltransferase 2D (MLL2), kinetochore localized astrin binding protein (C15orf23), remained as before (Table 2). In addition, the immunohistochemical distribution of PD-L1 protein in the stomach showed negative results [combined positive score (CPS): 1, tumor proportion score (TPS) <1%]. Based on the above results, immunotherapy was not considered at present. In order to find an effective treatment for this patient, we constructed a mini patient-derived xenograft (miniPDX) mouse model for targeted drug screening, and the experimental protocol is shown in Table 3. We selected adavosertib plus olaparib based on TP53 p.F270C (15), and Trametinib based on KRAS amplification (16) with secondary drug resistance mutations. In addition, we are still testing whether the patient can benefit from crizotinib, as well as other chemotherapy treatments. The entire time from the onset of PD in the patient to the availability of drug susceptibility results in the miniPDX model took about 2 weeks. Based on the relative value-added results, the trametinib group had the best performance, with values below 50%, while the adavosertib plus olaparib group and crizotinib group showed only lower results than the trametinib group (Figure 2A). The changes in body weight during the administration of trametinib in mice also showed that trametinib treatment had a small effect on body weight and did not fluctuate much during the administration of the drug (Figure 2B). From April 22, 2022 to October 12, 2022, the patient has been treated with 2 mg trametinib monotherapy once a day, and had benefited with the efficacy evaluation of SD with a dramatic decrease in CA199 tumor markers. From April 22, 2022 to June 29, 2022, CA199 decreased from 322.4 to 182.4 U/mL, unfortunately with severe side effects, such as bloating, poor appetite, fatigue and constipation. After October, the patient went to another hospital for treatment, and was lost to follow-up. In summary, the GC patient with MET amplification acquired three years of PFS with crizotinib therapy. Subsequently, the patient benefited from trametinib screened from miniPDX mouse model for at least a few months.
Table 2
| Gene | c.HGVS | p.HGVS | Functional region | Allele frequency |
|---|---|---|---|---|
| CARD11 | c.455T>G | p.L152R | EX5 | 7.9% |
| MLL2 | c.6440C>T | p.A2147V | EX31 | 4.5% |
| C15orf23 | c.17C>T | p.A6V | EX1 | 4.2% |
| KRAS | Amplification | – | – | CN =6 |
| TP53 | c.809T>G | p.F270C | EX8 | 30.1% |
c.HGVS, description of coding DNA (c.) variants by human genome variation society (HGVS); p.HGVS, description of protein (p.) variants by HGVS. CN, copy number.
Table 3
| Project | Grouping | Protocol |
|---|---|---|
| Control | No | |
| 1 | Olaparib + adavosertib | Ada, 30 mpk, po, qd*7 + Ola, 100 mpk, po, qd*7 |
| 2 | Trametinib | Traetinib 1 mpk, po, qd*7 |
| 3 | Crizotinib | Crizotinib 50 mpk, po, qd*7 |
| 4 | 5-FU + CF + Oxa | 5-FU 25 mpk, ip, qd*5 + CF 50 mpk, ip, qw + Oxa 5 mpk, ip, qw |
| 5 | 5-FU + CF + Irino | 5-FU 15 mpk, ip, qd*5 + CF 50 mpk, ip, qw + Irino 50 mpk, ip, q4d*2 |
| 6 | Nab-pac + Apa | Apa 100 mpk, po, qd*7 + Nab-pac 20 mpk, iv, qd*5 |
| 7 | TAS102 (Trifluridine) | Trifluridine, 9 mpk, po, bid*7 |
mpk, mg/kg; po, oral; qd, once a day; 5-FU, 5-fluorouracil; CF, calcium folinate; Oxa, oxaliplatin; ip, intraperitoneal; qw, once a week; Irino, irinotecan; Nab-pac, nab-paclitaxel; Apa, apatinib; q4d, once every four days; iv, intravenous; bid, twice a day.

Discussion
As one of the leading contributors to global malignancies incidence and mortality worldwide, GC is usually diagnosed in advanced stages, and it often presents a poor prognosis (17). Overexpression of MET occurs frequently in GC and has been proposed as a potential predictive biomarker for targeted therapy (18).
Crizotinib is a small molecule inhibitor of anti-MET approved by the FDA for lung cancer treatment (19). However, it is not been approved by the FDA for GC patients with MET amplification. The French-crizotinib trial (NCT02034981) demonstrated that crizotinib showed encouraging results in MET-amplified GC patients (14). Therefore, crizotinib was recommended based on the high CN of MET gene, and the patient achieved a prolonged remission. Three years later, the patient developed resistance and tumor progression.
By this time, NGS results revealed MET amplification mutation no was longer detected after crizotinib administration in this GC patient, while KRAS amplification and TP53 gene mutations remained. KRAS amplification may indicate tumor progression, and the constitutive activation of RAS-RAF-MEK-ERK signaling pathway, which will promote tumor growth and proliferation. Data suggest that inhibitors of mitogen-activated protein kinase 1 (MEK1) and mitogen-activated protein kinase 2 (MEK2), which inhibit the KRAS downstream signaling pathway, may be a good choice for KRAS mutant as a therapeutic tool (20). Trametinib, a MEK1/2 inhibitor, inhibits cell proliferation by affecting the MAPK pathway, primarily through its action on MEK protein, a downstream signaling protein of RAS and Raf-1 proto-oncogene (RAF), so trametinib may also be effective against cancer types with RAS or RAF mutations (21). Although trametinib has been shown in most studies to work by inhibiting KRAS in lung cancer treatment, it is rarely reported that GC can benefit from trametinib.
As cancer development becomes more and more complex, the degree of control of the disease process by conventional drugs becomes increasingly unacceptable and even harmful to patients. As a result, customized treatments are becoming increasingly important. With the popularity of genetic testing, customized screening protocols are becoming more accessible, and even patient-derived tumour xenografts (PDTX) samples can be as accurate as 90% for drug efficacy and resistance rates (22-24). Based on the results of this case, trametinib did show good anti-GC effects, even though trametinib is not traditionally the best drug for treating GC.
This study reports a GC patient with MET amplification received crizotinib and obtained the PFS of 34 months. After three years of crizotinib treatment, the patient developed resistance and tumor progression. NGS showed that MET amplification disappeared, which is rare in patients with GC. Because the patient refused chemotherapy, miniPDX mouse model was performed after progression to screen suitable drugs, and finally the patient was given trametinib treatment according to drug sensitivity. The tumor marker CA199 decreased significantly over two months of trametinib monotherapy, and CT results on June 30, 2022, showed stable disease (data not shown). The case report showed that the patient benefited from crizotinib and trametinib respectively after first-line chemotherapy and provided a reference for the use of genetic testing to identify mutation sites and select treatment options.
Conclusions
In the case, the potential drug target of GC was identified through genetic testing and treated with crizotinib firstly. After drug resistance developed, NGS was reapplied again to identify some new mutation sites. Finally, the drug was used experimentally across indications and treated with trametinib from April 22, 2022 to October 12, 2022. This shows that genetic testing is becoming more widely accepted in cancer treatment today.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-118/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-118/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-118/coif). Z.L. and H.K. are employees of Geneplus-Beijing (Beijing, China). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. For the mouse model, all procedures were performed under a project license (No. 2021-KY-0192) granted by ethics board of Henan Cancer Hospital, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Machlowska J, Baj J, Sitarz M, et al. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci 2020;21:4012. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol 2022;29:1559-74. [Crossref] [PubMed]
- Recondo G, Che J, Jänne PA, et al. Targeting MET Dysregulation in Cancer. Cancer Discov 2020;10:922-34. [Crossref] [PubMed]
- Van Cutsem E, Karaszewska B, Kang YK, et al. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin Cancer Res 2019;25:2414-23. [Crossref] [PubMed]
- NCCN Guidelines Panel. NCCN Clinical Practice Guidelines in Gastric Cancer (2024 Version 4). NCCN 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Hou GX, Song BB. Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: A case report and literature review. Math Biosci Eng 2019;16:5923-30. [Crossref] [PubMed]
- Lee J, Kim ST, Kim K, et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov 2019;9:1388-405. [Crossref] [PubMed]
- Ye W, He L, Su L, et al. Case Report: Prompt Response to Savolitinib in a Case of Advanced Gastric Cancer With Bone Marrow Invasion and MET Abnormalities. Front Oncol 2022;12:868654. [Crossref] [PubMed]
- Mo HN, Liu P. Targeting MET in cancer therapy. Chronic Dis Transl Med 2017;3:148-53. [PubMed]
- Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol 2019;30:1985-91. [Crossref] [PubMed]
- Aparicio T, Cozic N, de la Fouchardière C, et al. The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSé-Crizotinib Program. Target Oncol 2021;16:381-8. [Crossref] [PubMed]
- Sahgal P, Huffman BM, Patil DT, et al. Early TP53 Alterations Shape Gastric and Esophageal Cancer Development. Cancers (Basel) 2021;13:5915. [Crossref] [PubMed]
- Yamasaki J, Hirata Y, Otsuki Y, et al. MEK inhibition suppresses metastatic progression of KRAS-mutated gastric cancer. Cancer Sci 2022;113:916-25. [Crossref] [PubMed]
- López MJ, Carbajal J, Alfaro AL, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol 2023;181:103841. [Crossref] [PubMed]
- Röcken C. Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol 2023;149:467-81. [Crossref] [PubMed]
- Lin JJ, Shaw AT. Recent Advances in Targeting ROS1 in Lung Cancer. J Thorac Oncol 2017;12:1611-25. [Crossref] [PubMed]
- Punekar SR, Velcheti V, Neel BG, et al. The current state of the art and future trends in RAS-targeted cancer therapies. Nat Rev Clin Oncol 2022;19:637-55. [Crossref] [PubMed]
- Chesnokov MS, Khan I, Park Y, et al. The MEK1/2 Pathway as a Therapeutic Target in High-Grade Serous Ovarian Carcinoma. Cancers (Basel) 2021;13:1369. [Crossref] [PubMed]
- Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 2012;9:338-50. [Crossref] [PubMed]
- Cheng Y, Qin SK, Li J, et al. A multicenter clinical study: personalized medication for advanced gastrointestinal carcinomas with the guidance of patient-derived tumor xenograft (PDTX). J Cancer Res Clin Oncol 2022;148:673-84. [Crossref] [PubMed]
- Wang J, Huang J, Wang H, et al. Personalized Treatment of Advanced Gastric Cancer Guided by the MiniPDX Model. J Oncol 2022;2022:1987705. [Crossref] [PubMed]
Cite this article as: Xu W, Nie C, Wang H, Lv H, Chen B, Wang J, Wang S, Zhao J, He Y, Li Z, Kang H, Chen X. Gastric cancer patient with MET amplification treated with crizotinib achieves long-term survival: a case report. AME Case Rep 2025;9:27.


