
Uterine cystic adenomyosis: a case report
Highlight box
Key findings
• We report a rare case of uterine cystic adenomyosis (CA) lesion penetrating the myometrium, with symptoms of mild dysmenorrhea and irregular vaginal bleeding. Diagnosis was aided by B-ultrasound, magnetic resonance imaging (MRI), and cancer antigen 125 (CA125). Surgical resection followed by GnRH-a therapy improved symptoms and quality of life while preserving reproductive function.
What is known and what is new?
• Uterine CA is a unique form of adenomyosis with the fused or single lumen of the cystic space having a diameter greater than 1 cm that typically results in progressively worsening dysmenorrhea.
• This is the first reported case of an A1–B1 mixed type CA that was successfully treated by laparoscopic surgery supplemented with GnRH-a consolidation therapy.
What is the implication, and what should change now?
• The exclusive use of laparoscopic surgery may not be sufficient for patients with CA, particularly for young women. Further clinical trials investigating the efficacy of laparoscopic surgery in combination with GnRH-a consolidation therapy are needed to inform clinical practice and facilitate the transition towards personalized treatment approaches.
Introduction
Adenomyosis is a commonly diagnosed benign gynecologic condition characterized by infiltration of the myometrium by endometrial tissue composed of glands and stroma that often elicit hyperplasia and hypertrophy of surrounding smooth muscle cells (1). The condition is most prevalent among women of reproductive age, with a peak incidence between 40 and 50 years of age (1,2). The disease may be broadly classified as diffuse and focal adenomyosis (3). The occurrence of diffuse adenomyosis is relatively common while focal adenomyosis lesions, particularly those in the vicinity of the cystic uterine glands, are less frequently observed (4). When the diameter of the single or fused lumen of the cystic space exceeds 1 cm, the condition is termed cystic adenomyosis (CA) (3), also known as cystic adenomyoma or adenomyotic cyst (5). So far, less than 50 cases of CA have been reported in the literature (6).
Guided by general principle for treating adenomyosis, the specific treatment principle for CA highlights complete removal of the lesion, improvement of fertility and prevention of recurrence (7). As a special type of adenomyosis, CA patients may have remnant ectopic endometrial tissues after surgery. Therefore, taking effective measures, such as resection of the adenomyoma and restoration of the normal shape of the uterus, is necessary and crucial to minimize recurrence (8). Thorough removal of the lesion with minimal residual tissues, along with GnRH-a consolidation therapy and the levonorgestrel-releasing intrauterine system (LNG-IUS), may effectively halt recurrence and improve prognosis. In most cases, the prognosis and pregnancy outcomes of CA remained unclear, and therefore further studies are warranted. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-143/rc).
Case presentation
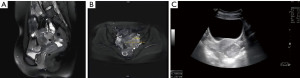
A 19-year-old patient had a body mass index (BMI) of 26 kg/m2 (170 cm height, 75 kg weight), with no history of sexuality, childbearing, prior surgeries, smoking nor alcohol abuse, and no family history of any genetic disorders. She had regular menstruation, with a 30-day menstrual cycle, and 7 days of mild dysmenorrhea. In April 2023, she sought medical attention at a local hospital due to irregular vaginal bleeding that lasted for more than one month. Since the patient was not sexually active, a bimanual gynecological examination was not performed, and no abnormalities were identified during anal examination. A transabdominal gynecological ultrasound was initially conducted, which indicated the presence of a pelvic mass, followed by the performance of a further pelvic magnetic resonance imaging (MRI) scan. The enhanced pelvic MRI revealed a round cystic mass behind the uterus with a blood accumulation signal (Figure 1A,1B). Therefore, the patient was referred to the Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University for further diagnosis and treatment. A hypoechoic nodule measuring approximately 4.8 cm × 3.9 cm × 4.9 cm was observed in the muscle wall of the posterior uterine wall by using transabdominal B-ultrasound. The boundary was clear, but the internal echo was not uniform, and the inner membrane was slightly displaced (Figure 1C). It is important to diagnostically differentiate CA from other gynecological conditions. For instance, cystic degeneration of uterine fibroid typically presents with a history of uterine fibroids and an ultrasound showing a permeable cystic cavity. In this case, the patient lacked a history of uterine fibroids and the cystic cavity was poorly permeable, which did not align with the above characteristics and was therefore not considered as cystic degeneration of uterine fibroid. In addition, adnexal cysts such as ovarian or fallopian tube cysts can sometimes be difficult to be distinguished from the uterine cysts by imaging examination. The preoperative diagnosis was concluded as a pelvic mass with a cancer antigen 125 (CA125) level of 51.48 U/mL, a value that exceeds the normal range of 0–35 U/mL.
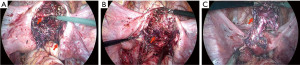
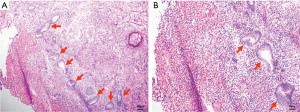
After admission, a series of examinations were conducted to avoid surgical contraindications which included a pelvic magnetic resonance scan conducted at an external hospital in consultation with an imaging specialist, an electrocardiogram, a chest X-ray, immune indicators, coagulation function tests, biochemistry tests, and routine blood tests. Given the patient’s lack of sexual activity, no uterine operations were conducted. Prior to the procedure, the patient was fasted and consumed laxatives for bowel preparation for a period of 12 hours. Laparoscopic myomectomy resection and repair was performed under general anesthesia on May 8, 2023. During operation, dense adhesions were found among a portion of the colorectum, omentum, uterus and bilateral adnexal areas. A cystic mass of approximately 5.0 cm × 5.0 cm × 4.0 cm was visible in the posterior wall of the uterus, which was enlarged to the size of 7.0 cm × 5.0 cm × 4.0 cm. Upon opening the capsule (the serous layer of the posterior uterine wall), thick, chocolate-like fluids flowed out (Figure 2). A sinus of approximately 1.0 cm in diameter was found to be connected to the uterine cavity at the lower posterior wall of the uterus, and the cystic wall was attached to the endometrium. Additionally, a cord-shaped endometriotic lesion was visible on the surface of the uterosacral ligament on both sides. The sutures were applied intermittently and reinforced, and the uterus was sutured in two layers, with the first layer being sutured with 2-0 absorbable threads and the second layer sutured with 1-0 absorbable threads. Postoperative pathology revealed adenomyosis with smooth muscle hyperplasia and unusual spindle cell hyperplasia in the posterior uterine wall lesions (Figure 3). The discharge diagnosis was concluded as CA and female pelvic adhesion. After three cycles (21 days per cycle) of goserelin injection, administered via subcutaneous injection at a dosage of 3.6 mg, MRI enhancement and B-ultrasound indicated that the uterus was small and had a uniform myometrial echo (Figure 4). The patient’s CA125 level was 9.90 U/mL. The patient resumed her menstrual cycles after drug withdrawal. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
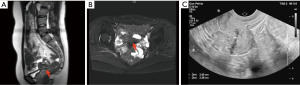
In a follow-up appointment, the patient was advised to take strict contraceptive measures for a period of two years. She was instructed to have repeat ultrasound of the pelvis every 6 months to a year, and if necessary, an MRI of the pelvic to determine the thickness of myometrium. She was also referred to a preconception fertility clinic for an assessment of uterine fertility prior to conception.
Discussion
Adenomyosis is a lesion caused by hyperplastic expansion of the endometrium, including glands and stroma, into the myometrium. It is mainly manifested by excessive menstruation, pelvic pain, or infertility (2). Although the pathological cause of adenomyosis remains unclear, two widely proposed theories are invagination and metaplasia (9). Invagination theory, including the tissue damage and repair hypothesis, suggests that endometrial invasion into the myometrium is caused by tissue damage and repair processes, while the metaplastic theory proposes that the ectopic endometrium is formed by a remnant of Mullerian ducts or stem cells. Based on the imaging findings, adenomyosis can be classified into diffuse adenomyosis, focal adenomyosis, and other special type of adenomyosis. Focal adenomyosis comprises uterine adenomyoma and uterine CA (10,11).
The diagnostic criteria for CA are as follows: (I) presence of an isolated cystic lesion; (II) normal uterine endometrial cavity, fallopian tube and ovary; (III) surgical removal of the cystic mass for pathological examination; (IV) capsule lining endometrial epithelium, glands, and stromal components; (V) presence of chocolate-like brown liquid content; and (VI) absence of adenomyosis, except for a small amount in the myometrium adjacent to the accessory cavity (12). It is important to distinguish CA from other conditions such as obstructive uterine malformation, uterine fibroids with cystic changes, adnexal mass, and uterine adenomyosis lesions with internal hemorrhage. Pathological sections are often used to confirm the diagnostic results (13,14).
The basic principle for treating CA is to remove the lesion, improve fertility, and prevent recurrence. Unlike diffuse adenomyosis, CA lesions are more clearly distinguished from the normal myometrium. Surgical resection of the lesion can completely or partially resolve the pain and have a positive impact on fertility (14). Although there is no clear evidence that postoperative hormone therapy can reduce the recurrence rate of the disease (13), given that CA is a type of adenomyosis, the use of GnRH-a after surgery may improve the surgical outcome and alleviate postoperative dysmenorrhea symptoms (15).
Based on the B-ultrasound, MRI, and intraoperative results, no malformations in the uterus, fallopian tubes, or ovaries were seen, and the morphology was normal. A cystic mass of 5 cm in diameter was excised, and thick chocolate-like fluids leaked from the cyst. The pathological sections of the cystic wall showed ectopic endometrium and spindle cells, supporting its diagnosis as CA. Based on the criteria of MUSCLE (myometrial location, uterine site, structure, contents, level, endometrial or inner lining), five subtypes were classified for all CA cases thus far, namely, submucous or intramural cystic adenoma (A1), cystic polypoid lesions (A2), subserous cystic adenomas (B1), exophytic growth (B2), and uterine-like masses within the uterus (C) (16), with no mention of any previous case of CA lesions penetrating inwardly into the uterine cavity of the patients. Therefore, the current case is classified as an A1–B1 mixed type CA, which complements the existing classification criteria for CA. The patient’s lesion crossed the entire uterine cavity and differed from the ‘normal endometrial cavity’ in the diagnostic criteria for CA. Such phenomenon could be attributed to the following mechanisms: (I) during the development of the Mullerian ducts, fusion anomaly occurs, and the endometrium extends deep into the myometrium of the uterus (17). (II) After menarche, the internal epithelial cycle exfoliates in the myometrium to form a hemorrhagic cystic cavity. The cyst gradually increases and communicates with the uterine cavity, extending outwardly to the subserosal layer. Further investigation is warranted to find out the mechanisms underlying this special type of CA. To meet the patient’s need for pregnancy, laparoscopic surgery was performed to close the lesion and strengthen the muscle layer, preventing uterine rupture during pregnancy caused by the muscle layer defect of the posterior uterine wall (18). Following surgery, GnRH-a drug-induced amenorrhea was used for 3 months to prevent recurrence (19).
Conclusions
In conclusion, we report a rare case in which the CA lesion penetrates the myometrium. It differs from conventional CA in that the patient had mild dysmenorrhea, mainly irregular vaginal bleeding. B-ultrasound, MRI, and CA125 are important auxiliary means in the diagnosis of this disease. Surgical resection of the lesion supplemented by GnRH-a consolidation therapy relieved her symptoms and improved her quality of life while preserving her reproductive function.
The exclusive use of laparoscopic surgery may not be sufficient for patients with CA, particularly for young women. Further clinical trials investigating the efficacy of laparoscopic surgery in combination with GnRH-a consolidation therapy are needed to inform clinical practice and facilitate the transition towards personalized treatment approaches.
Acknowledgments
The authors thank Dr. Yingjie Wang, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, for his helpful comments on the manuscript.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-143/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-143/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-143/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril 2018;109:369-70. [Crossref] [PubMed]
- Schrager S, Yogendran L, Marquez CM, et al. Adenomyosis: Diagnosis and Management. Am Fam Physician 2022;105:33-8. [PubMed]
- Harada T, Khine YM, Kaponis A, et al. The Impact of Adenomyosis on Women's Fertility. Obstet Gynecol Surv 2016;71:557-68. [Crossref] [PubMed]
- Manta L, Suciu N, Constantin A, et al. Focal adenomyosis (intramural endometriotic cyst) in a very young patient - differential diagnosis with uterine fibromatosis. J Med Life 2016;9:180-2. [PubMed]
- Cucinella G, Billone V, Pitruzzella I, et al. Adenomyotic cyst in a 25-year-old woman: case report. J Minim Invasive Gynecol 2013;20:894-8. [Crossref] [PubMed]
- Xu T, Li Y, Jiang L, et al. Subserous Cystic Adenomyosis: A Case Report and Review of the Literature. Front Surg 2022;9:807676. [Crossref] [PubMed]
- Kwack JY, Im KS, Kwon YS. Conservative surgery of uterine adenomyosis via laparoscopic versus laparotomic approach in a single institution. J Obstet Gynaecol Res 2018;44:1268-73. [Crossref] [PubMed]
- Kwack JY, Kwon YS. Laparoscopic Surgery for Focal Adenomyosis. JSLS 2017;21:e2017.00014.
- Guo SW. Cracking the enigma of adenomyosis: an update on its pathogenesis and pathophysiology. Reproduction 2022;164:R101-21. [Crossref] [PubMed]
- Van den Bosch T, de Bruijn AM, de Leeuw RA, et al. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol 2019;53:576-82. [Crossref] [PubMed]
- Kishi Y, Suginami H, Kuramori R, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol 2012;207:114.e1-7. [Crossref] [PubMed]
- Acién P, Bataller A, Fernández F, et al. New cases of accessory and cavitated uterine masses (ACUM): a significant cause of severe dysmenorrhea and recurrent pelvic pain in young women. Hum Reprod 2012;27:683-94. [Crossref] [PubMed]
- Takeuchi H, Kitade M, Kikuchi I, et al. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: a review of nine cases. Fertil Steril 2010;94:862-8. [Crossref] [PubMed]
- Protopapas A, Kypriotis K, Chatzipapas I, et al. Juvenile Cystic Adenomyoma vs Blind Uterine Horn: Challenges in the Diagnosis and Surgical Management. J Pediatr Adolesc Gynecol 2020;33:735-8. [Crossref] [PubMed]
- Zhang L, Guo Z, Pang Y, et al. Cystic adenomyoma of the uterus: Case report and literature review. Open Life Sci 2024;19:20220846. [Crossref] [PubMed]
- Brosens I, Gordts S, Habiba M, et al. Uterine Cystic Adenomyosis: A Disease of Younger Women. J Pediatr Adolesc Gynecol 2015;28:420-6. [Crossref] [PubMed]
- Kerbage Y, Dericquebourg S, Collinet P, et al. Cystic adenomyoma surgery. J Gynecol Obstet Hum Reprod 2022;51:102313. [Crossref] [PubMed]
- Zhao CZ, Wang B, Zhong CY, et al. Management of uterine cystic adenomyosis by laparoscopic surgery: case report. BMC Womens Health 2021;21:263. [Crossref] [PubMed]
- Chao X, Song X, Wu H, et al. Adjuvant therapy in conservative surgery for adenomyosis. Int J Gynaecol Obstet 2021;154:119-26. [Crossref] [PubMed]
Cite this article as: Ma X, Shen J, Tang R, Sun F, Chen W, Yang J. Uterine cystic adenomyosis: a case report. AME Case Rep 2025;9:23.


