
Case series of adult Wilms’ tumor and review of the literature
Highlight box
Key findings
• We reported four cases of treatment and 5-year follow-up data of adult Wilms’ tumor (AWT), which is rarely reported in the literature.
What is known and what is new?
• AWT is an extremely rare kidney malignancy in clinical practice and is very difficult to diagnose preoperatively. A unified treatment plan of AWT is lacking and the prognosis is unfavorable.
• The current evidence-based pathology, diagnosis, staging, treatment, prognosis, and mechanistic research of AWT have been reviewed in this article.
What is the implication, and what should change now?
• Early diagnosis and early treatment by successful tumor resection with standardized postoperative adjuvant therapy according to pathological typing and staging can significantly improve survival of AWT patients.
Introduction
Wilms’ tumor (WT) is one of the most common malignant tumors in children, with an incidence of about 1/7,000, occurring mainly before the age of 5 years, and mainly presenting as a painless, rapidly growing abdominal mass (1). Adult Wilm’s tumor (AWT) is very rare and refers to adults who develop it over the age of 15 years. The incidence of AWT is only 2% of all WT, and its first symptoms are mostly back pain and hematuria (2). The diagnosis, staging, and treatment of AWT are not yet standardized, and the current treatment process is based on the National Wilms’ Tumor Study Group (NWTSG), a multicenter group of experts from the United States and Europe, and the guidelines for the treatment of pediatric WT published by the International Society of Pediatric Oncology (SIOP).
The diagnosis of AWT mainly depends on postoperative pathological diagnosis, and its preoperative diagnosis is difficult, mainly relying on imaging [computed tomography (CT) or magnetic resonance imaging (MRI)] examination. Although the imaging findings are slightly different, it is difficult to completely distinguish them from other renal malignancies. Kilton et al. proposed in 1980 that the diagnosis of AWT must meet the following criteria based on pathology: (I) primary renal tumor; (II) primitive maternal cell-like round or spindle cell component; (III) immature or embryonic tubular or glomerular-like structure formation; (IV) absence of renal cancer tissue; (V) clear histological images; and (VI) age over 15 years (3). This criterion is still used today, but only about 400 cases have been reported under this criterion. The staging of NWTSG and SIOP for WT exhibited similarities, encompassing stages I to V. AWT presents with a higher malignancy grade and advanced stage, rendering it susceptible to recurrence and metastasis at a ratio of up to 29%. The prognosis is unfavorable, with a 30% of the 3-year survival rate (2).
Because AWT has a lower incidence and different clinical characters from children with WT, there is no widespread consensus on its diagnosis and treatment. In this study, the clinical features, treatment, and prognostic outcomes of AWT patients recruited from the Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology from August 2010 to January 2019 were analyzed. At the same time, the current diagnosis, treatment, and research progress of AWT were comprehensively summarized by reviewing the existing literature. We present this article in accordance with the AME Case Series reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-208/rc).
Case presentation
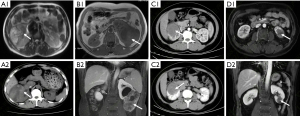
There were four AWT cases without special medical history, including three males and one female, aged from 29 to 51 years old. One patient was hospitalized with lumbar and abdominal distension. One patient came to the hospital after a space-occupying lesion of the kidney found during a routine physical examination. The other two patients came with lumbar pain accompanied by painless gross hematuria. Preoperative imaging examinations (Figure 1) showed uneven-density renal tumor lesions. One case showed a rupture of a massive tumor in the right kidney with perirenal hematoma and inferior vena cava thrombus of Mayo grade III. There was no tumor metastasis in the other three cases. One patient underwent radical nephrectomy with vena cava tumor embolus removal. Two patients underwent radical nephrectomies, and another one underwent partial nephrectomy.
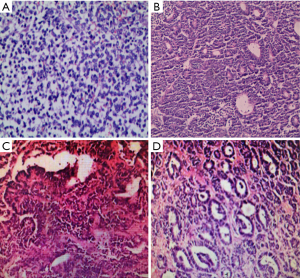
All tumors of the four cases were successfully removed, and the postoperative pathology indicated them to be WT with negative incisal margins. The pathological HE staining pictures are shown in Figure 2. Case 1 was a high-grade small round cell sarcoma with extensive hemorrhagic necrosis and invasion of perirenal adipose tissue. It was considered a germinal AWT tumor with total extraction of the inferior vena cava tumor thrombus. Immunohistochemistry staining showed positive of WT-1, CD99, CD117 and VIM, with rapid tumor proliferation by high Ki67 expression. Case 2 was a mixed-type AWT combined with a partial posterior renal adenoma component. The tumor is breaking through the capsule. Immunohistochemistry results showed positive staining of WT-1, Bcl-2, PCK, and VIM, with an 80% positive rate of Ki67. Case 3 was a complete epithelial AWT with hemorrhagic infarction, without breaking through of the capsule. Immunohistochemistry staining showed positive expression of PCK, VIM, PAX-8, PAX-2, CAIX, luminal margin cathepsinK, SDHB, partial WT-1, focal CD117, and CD56, with 1% level of Ki67. Case 4 had epithelial AWT with necrosis and rupture but did not invade perirenal fat. Immunohistochemistry results showed positive for PAX-8, PAX-2, WT-1, CD56, CD10, PCK, EMA, MCM2 (30% regional positive), and 25% expression level of Ki67. According to NWTSG staging criteria, two cases were stage I, one case was stage II, and one case was stage III.
All cases were followed up over 5 years, and the postoperative imaging examinations are shown in Figure 3. Currently, cases 1 and 2 have died, while cases 3 and 4 have survived. Case 1 refused radiotherapy and chemotherapy after surgery. Reexamination at 3 months after surgery revealed multiple metastases in bilateral lungs, liver, and right anterior branch of the portal vein. The patient gave up radiotherapy and chemotherapy and died 9 months after surgery. In case 2, chemotherapy was started 2 weeks after surgery. During the 1–2 cycles, the actinomycin D + vincristine regimen was used. Due to the side effect of IV-degree thrombocytopenia and granulocytopenia, the treatment plan during 3–7 cycles was changed into the pirarubicin + vincristine regimen. Fifteen months after surgery, the tumor recurred at the left side of the pelvic cavity, invading the colon and peripheral lymph node. He died 19 months later, with an overall survival of 36 months. The remaining two cases survived. Case 3 did not receive adjuvant therapy, and no recurrence or metastasis was observed during the 70 months follow-up. Case 4 started chemotherapy 4 weeks after surgery, with an etoposide + carboplatin regimen in cycles 1–2, cyclophosphamide + vincristine + adriamycin in cycles 3–4, and etoposide + carboplatin in cycles 5–7, during which the dose of chemotherapy drugs was reduced due to Grade IV myelosuppression. The postoperative CT scan suggested negative recurrence and metastasis. The baseline, diagnosis, treatment, and prognosis data for all cases are shown in Table 1.
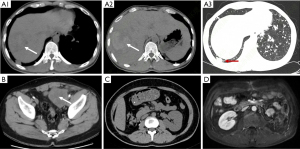
Table 1
| Perioperative characters | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age (years) | 38 | 37 | 27 | 51 |
| Gender | Male | Male | Female | Male |
| Initial symptoms | Right-sided back pain with hematuria | None | Right lumbar swelling | Left-sided back pain with hematuria |
| Imaging data | ||||
| Tumor location | Middle and lower pole of right kidney | Middle and lower pole of the left kidney | Upper pole of the right kidney | Middle and lower pole of the left kidney |
| Size (cm×cm) | 10×7 | 13×7 | 5×5 | 4×3 |
| Metastasis | Inferior vena cava tumor embolus | None | None | None |
| Surgical method | Radical right nephrectomy + removal of inferior vena cava tumor embolus | Radical left nephrectomy | Posterior laparoscopic radical right nephrectomy | Robot-assisted laparoscopic partial left nephrectomy |
| Pathology | ||||
| Type | AWT (germinal type) with vena cava tumor thrombosis | Partly AWT (mixed) conformation, partly adenoma conformation | Partly AWT (mixed) conformation, partly adenoma conformation | AWT (epithelial type) with necrosis and rupture |
| Cutting edge | – | – | – | – |
| NWTSG staging | III | II | I | I |
| Creatinine (μmol/L) | 151 | 118 | 91 | 117 |
| eGFR (mL/min/1.73 m2) | 45.1 | 44.2 | 73.7 | 61.8 |
| Post-operative adjuvant therapy | ||||
| Chemotherapy plan | None | Cycles 1–2: actinomycin D + vincristine cycle 3–7: pyridoxine + vincristine | None | Cycles 1–2: etoposide + carboplatin; cycles 3–4: cyclophosphamide + vincristine + adriamycin; cycles 5–7: etoposide + carboplatin |
| Initial treatment after surgery (days) | 15 | 31 | ||
| PFS (months) | 3 | 15 | >70 | >68 |
| Recurrent metastases | Both lungs, liver, portal vein | Pelvic, peri-colonic lymph nodes | None | None |
| OS (months) | 9 | 36 | >70 | >68 |
AWT, adult Wilms’ tumor; PFS, progression-free survival; OS, overall survival.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
AWT is very rare and can occur in adults at any age. The current literature on AWT is mainly presented by case reports, with very limited reports including large samples (4). Hu et al. summarized the literature on reported AWT from 2004 to 2016, with ages 19–54 years (mean age 35 years), which showed no significant gender differences, and tumor diameters ranging in size from 4–15 cm (5). The diameters of tumors sizes in our study are in accordance with the data.
Pathology
There was no disparity in the pathologic manifestation of AWT and pediatric WT. There are three prevalent histologic types of AWT: germinal, epithelial, and mesenchymal, which can exist independently or in combination. WT with the germinal type is more aggressive than the other two types of tumor cells and indicates a poor prognosis. In addition to the six diagnostic criteria proposed by Kilton based on pathological findings, positive immunohistochemical tests for cytokeratin, vimentin, desmin, actin, and WT-1 can assist in distinguishing other rare renal tumors, such as renal sarcoma, mesangial renal tumor, clear cell sarcoma, or rhabdomyosarcoma. Among them, the positivity of WT-1 suggests the existence of germinal or proliferating epithelial tissue, which is not expressed in mature epithelial and mesenchymal tissues (6).
Diagnosis
Since AWT does not exhibit significant differences in symptoms and signs compared to other types of renal tumors, the preoperative assessment primarily utilizes renal B-ultrasound, enhanced CT, and MRI, making it arduous to confirm the diagnosis (7). B-ultrasound, serving as a preliminary screening method, can disclose tumor size, shape, and manifestations of the residual kidney and detect cancer thrombus, yet it is unable to characterize the masses. IVU can demonstrate an enlarged renal shadow, elongation, deformation, displacement, or damage of the renal pelvis calyces. After following up on CT and MRI data from 16 cases of AWT, Wu et al. attempted to discover the specific image manifestations of AWT and proposed that CT scans typically show a solid or solid cystic mass of uneven density (8). On enhanced CT, AWT usually manifests heterogeneity and a slightly slower contrast regression compared to normal renal parts, but this presentation cannot distinguish between renal collecting duct carcinoma, renal suspensory cell carcinoma, and renal lymphoma. In MRI, AWT emerges as isointense or hypointense foci on the T2W phase and is usually smaller than normal renal parenchyma in all phases of enhancement. AWT is generally large, often disrupts the renal contour, and might present as isointense or hypoinsense signal on the T2W phase due to iron-containing heme deposits, which can be employed to distinguish renal clear cell carcinoma, cystic kidney cancer, and renal cysts that present as high signal on the T2W phase (9). Large-volume renal cell carcinoma frequently shows central tumor necrosis and calcification and thus may exhibit an inhomogeneous high signal on the T2W phase. Lymph node or distal metastasis may occur in up to 10% of cases. Molecular testing may help exclude other types of malignancies (10).
Staging
NWTSG and SIOP classified WT into stages I–V according to tumor pathology. Stage I: the tumor is restricted within the kidney, does not invade the renal envelope, and can be completely resected, with no invasion of the renal sinus vascular and no ureteral wall invasion. Stage II: the tumor exceeds the boundaries of the kidney or breaks through the pseudo-envelope; maybe it invades the renal sinus vasculature and lymphatics; maybe it invades adjacent organs or the vena cava; but it can still be completely resected. Stage III: residual tumor tissue (including unresectable tumor with positive incisal margins, tumor rupture contaminating the abdominal cavity, residual lymph nodes with local metastasis, residual vascular tumor plugs, puncture biopsy performed before surgery, or neoadjuvant chemotherapy). Stage IV: hematogenous metastasis (to the lung, liver, bone, or brain) or distant lymph node metastasis outside the abdominopelvic and pelvic cavities. Stage V: tumor involving bilateral kidneys. Stages III and IV accounted for 30% and 50% of childhood and adult WT, respectively (2).
Treatment
There is as yet no widely accepted treatment protocol for AWT. Some patients are treated based on the approach for pediatric WT. Currently, most scholars advocate a comprehensive treatment plan integrating surgery, radiotherapy, and chemotherapy in accordance with the pathological stage (11).
According to SIOP, radical nephrectomy is preferred for unilateral WT (involving the perinephric fascia, perinephric fat, the affected kidney, the ipsilateral adrenal gland, the abdominal aorta or paraval lymph nodes extending from the foot of the diaphragm to the bifurcation of the abdominal aorta or the inferior vena cava, and the ureter above the bifurcation of the iliac vessels). However, partial nephrectomy is recommended only for contralateral renal disease, hereditary WT high-risk patients, and individuals with a solitary kidney (12). Lymph node dissection can reduce the local recurrence rate and lengthen the survival time of AWT (13). Common postoperative complications encompass intestinal obstruction, wound infection, vascular injury, and other organ damage. The risk factors for surgical complications include the formation of an inferior vena cava or intra-atrial tumor embolus and a tumor diameter greater than 10 cm (14). Whether the operation is thorough is also directly related to the prognosis of the patient. As long as the condition permits, the primary lesion should be removed as soon as feasible and as completely as possible.
The most common chemotherapeutic agents currently considered for the treatment of WT are actinomycin D, vincristine, adriamycin, cyclophosphamide, isocyclophosphamide, etoposide, and carboplatin. For chemotherapy modality, NWTSG recommends it after resection of the primary tumor, while SIOP recommends neoadjuvant chemotherapy 4 weeks prior to tumor resection with the aim of reducing the risk of intraoperative tumor rupture, but may affect the true clinical staging (15). NWTSG recommends various postoperative combination chemotherapy regimens according to their staging. For patients with stages I–II, two drugs are recommended for combination chemotherapy, which can effectively improve patients’ postoperative survival. For stage III–IV, patients need to add adriamycin and abdominal radiotherapy to the above treatment. The overall efficiency of the first-line drug ICE chemotherapy regimen (isocyclophosphamide, carboplatin, and etoposide) exceeded 80%, with 27% of patients getting complete remission and 55% getting partial remission (16). Compared to pediatric WT, AWT is less sensitive to chemotherapy, and chemotherapy-related toxicities are higher, probably related to disease stage and chemotherapy dose (17). However, one case report showed immunotherapy and anti-angiogenesis combined with chemotherapy is promising new approaches for treating AWT (18).
WT is also more sensitive to radiotherapy, and thus, radiotherapy is an essential component of the comprehensive treatment. Currently, radiotherapy is mainly employed for advanced-stage WT (stages III, IV, and V) or early-stage WT with unfavorable histology. Long-term complications include radiation nephritis and radiation-induced spinal nerve root damage. The recommended chemoradiotherapy regimens by NWTSG and SIOP in different stages of WT are shown in Table 2. For recurrent AWT, dual BRAF/MEK-targeted therapy might have a robust response (19).
Table 2
| Staging | NWTSG | SIOP | |||||
|---|---|---|---|---|---|---|---|
| Chemotherapy | Radiotherapy | Chemotherapy | Radiotherapy | ||||
| Pre-operative | Post-operative | ||||||
| I | 18 weeks of VA plan | – | 4 weeks of VA plan | 4 weeks of VA plan | – | ||
| II | 18 weeks of VA plan | – | 4 weeks of VA plan | 27 weeks of VDA plan | Without nodules: –; with nodules: 15 Gy | ||
| III | 24 weeks of VDA plan | 10.8 Gy | 4 weeks of VA plan | 27 weeks of VDA plan | 15 Gy | ||
| IV | 24 weeks of VDA plan | Pulmonary metastases: 12 Gy; abdominal metastases: 10.8 Gy | 6 weeks of VDA plan | Complete remission after 9 weeks: 27 weeks on VDA regimen; incomplete remission after 9 weeks: ICED regimen 34 weeks | Pulmonary lesions disappeared after 9 weeks: –; no disappearance of lung lesions after 9 weeks: 12 Gy | ||
A, actinomycin D; C, cyclophosphamide; D, adriamycin; E, etoposide; I, isocyclophosphamide; V, vincristine. NWTSG, National Wilms’ Tumor Study Group; SIOP, International Society of Pediatric Oncology; WT, Wilms’ tumor.
Prognosis
The prognosis of AWT is influenced by the tumor’s initial stage, histopathological structure, progression-free survival, treatment modality, and site of recurrence. In adults with advanced-stage WT, chemoradiotherapy presents low sensitivity and high toxicity, leading to a higher rate of metastasis compared to children (20). The 3-year survival rate of patients with recurrence stands at approximately 30%. The poor prognosis factors include staging beyond stage I, recurrence at the site of abdominal radiotherapy, recurrence within 12 months of initial treatment, and recurrence after three-drug chemotherapy.
Research
The molecular mechanism of AWT remains to be elucidated. Previous studies have indicated a hereditary link to the disease, with specific chromosome or DNA alterations associated with AWT. Deletion or mutation in the short arm region 13 (11p13) of chromosome 11 is significantly associated with the pathogenesis of AWT, suggesting that this region may harbor a crucial tumor suppressor gene for AWT (21). Subsequent investigations have revealed that the WT-1 gene within this region can influence AWT development as a transcriptional regulator possessing a unique DNA-binding domain. Aberrant expression patterns in mesenchymal embryonic stem cells may lead to differentiation disorder and sustained proliferation of immature renal tissue (22,23). Recent study by Wang et al. (24) has linked elevated levels of VEGF-A in serum and tissues to a poor prognosis in AWT, laying the groundwork for anti-angiogenic therapy as a potential novel treatment strategy.
Conclusions
AWT is a rare and challenging condition to diagnose preoperatively due to the lack of specific clinical manifestations. Imaging examinations often lead to misdiagnosis from other types of kidney tumors, necessitating confirmation through pathological examination. Early tumor staging and standardized, comprehensive treatment are key points to improve patient prognosis. However, there is currently no established treatment guideline for AWT, leading to reliance on pediatric WT guidelines despite significant differences between the two situations. Therefore, there is an urgent need for more studies on the pathogenesis and treatment of AWT.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-208/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-208/prf
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-208/coif). Y.L. reports that this research was supported by the Natural Science Foundation of Hubei Province (No. 2022CFB477) and Tongji Hospital (No. 2023A10). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilms Tumor and Other Childhood Kidney Tumors Treatment (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD); 2002.
- Huszno J, Starzyczny-Słota D, Jaworska M, et al. Adult Wilms' tumor - diagnosis and current therapy. Cent European J Urol 2013;66:39-44. [Crossref] [PubMed]
- Kilton L, Matthews MJ, Cohen MH. Adult Wilms tumor: a report of prolonged survival and review of literature. J Urol 1980;124:1-5. [Crossref] [PubMed]
- Kattan J, Tournade MF, Culine S, et al. Adult Wilms' tumour: review of 22 cases. Eur J Cancer 1994;30A:1778-82. [Crossref] [PubMed]
- Hu J, Jin LU, He T, et al. Wilms' tumor in a 51-year-old patient: An extremely rare case and review of the literature. Mol Clin Oncol 2016;4:1013-6. [Crossref] [PubMed]
- Chapman S, Lichtbroun B, Patel H, et al. Epithelial Predominant Wilms Tumor in an Adult Patient: Case Report and Literature Review. J Kidney Cancer VHL 2024;11:33-9. [Crossref] [PubMed]
- Bajaj S, Gandhi D, Shah J, et al. Adult Wilms tumor: An unusual case report with dedicated literature review. Clin Imaging 2022;83:138-43. [Crossref] [PubMed]
- Wu J, Zhu Q, Zhu W, et al. CT and MRI imaging features and long-term follow-up of adult Wilms' tumor. Acta Radiol 2016;57:894-900. [Crossref] [PubMed]
- Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J Clin Oncol 2001;19:1926-34. [Crossref] [PubMed]
- Chan GJ, Stohr BA, Osunkoya AO, et al. Wilms Tumor: An Unexpected Diagnosis in Adult Patients. Arch Pathol Lab Med 2024;148:722-7. [Crossref] [PubMed]
- Sharma A, Shaikh I, Chaudhri R, et al. Adult Wilms' Tumour. J Clin Diagn Res 2016;10:PJ01-2. [PubMed]
- Long CJ, Mittal S, Kolon TF. Expanding the Use of Nephron-Sparing Surgery for Wilms Tumor. J Natl Compr Canc Netw 2022;20:540-6. [Crossref] [PubMed]
- Zhuge Y, Cheung MC, Yang R, et al. Improved survival with lymph node sampling in Wilms tumor. J Surg Res 2011;167:e199-203. [Crossref] [PubMed]
- Ritchey ML, Shamberger RC, Haase G, et al. Surgical complications after primary nephrectomy for Wilms' tumor: report from the National Wilms' Tumor Study Group. J Am Coll Surg 2001;192:63-8; quiz 146. [Crossref] [PubMed]
- de Kraker J, Graf N, van Tinteren H, et al. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms' tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet 2004;364:1229-35. [Crossref] [PubMed]
- Abu-Ghosh AM, Krailo MD, Goldman SC, et al. Ifosfamide, carboplatin and etoposide in children with poor-risk relapsed Wilms' tumor: a Children's Cancer Group report. Ann Oncol 2002;13:460-9. [Crossref] [PubMed]
- Reinhard H, Aliani S, Ruebe C, et al. Wilms' tumor in adults: results of the Society of Pediatric Oncology (SIOP) 93-01/Society for Pediatric Oncology and Hematology (GPOH) Study. J Clin Oncol 2004;22:4500-6. [Crossref] [PubMed]
- Zhao Q, Xiong Q, Song Q. Metastatic adult Wilms' tumor managed by chemotherapy, immunotherapy and target therapy: a case report. Future Sci OA 2024;10:FSO915. [Crossref] [PubMed]
- Kroll MR, Au C, Slostad J, et al. Case report: Metastatic BRAF V600E-mutated adult Wilms' tumor with robust response to BRAF/MEK inhibitor therapy. Front Oncol 2024;14:1376270. [Crossref] [PubMed]
- Sakthivel V, Adeeb IZ, Vijayabalan D. Recent Improvements in Adult Wilms Tumor Diagnosis and Management: Review of Literature. J Kidney Cancer VHL 2023;10:32-6. [Crossref] [PubMed]
- Akpa MM, Iglesias DM, Chu LL, et al. Wilms tumor suppressor, WT1, suppresses epigenetic silencing of the β-catenin gene. J Biol Chem 2015;290:2279-88. [Crossref] [PubMed]
- Wagner KD, Cherfils-Vicini J, Hosen N, et al. The Wilms' tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun 2014;5:5852. [Crossref] [PubMed]
- Park M, Choi Y, Choi H, et al. Wilms' tumor suppressor gene (WT1) suppresses apoptosis by transcriptionally downregulating BAX expression in immature rat granulosa cells. J Ovarian Res 2014;7:118. [PubMed]
- Wang J, Fan S, Feng Y, et al. Antiangiogenic therapy for Wilms tumor in an adult and literature review. Anticancer Drugs 2019;30:640-5. [PubMed]
Cite this article as: He Y, Yang F, Gao Q, Xiang Y, Chen Z, Chen X, Luan Y. Case series of adult Wilms’ tumor and review of the literature. AME Case Rep 2025;9:39.


