
Individualized treatment of pregnancy-associated breast cancer: a report of two cases and literature review
Highlight box
Key findings
• Pregnancy-associated breast cancer (PABC) occurs during the special physiological period of female pregnancy, requiring a clinical approach that considers both the effectiveness of maternal treatment and the safety of the baby, creating a clinical challenge.
What is known and what is new?
• PABC occurs during the special physiological period of female pregnancy, requiring a clinical approach that considers both the effectiveness of maternal treatment and the safety of the baby, creating a clinical challenge.
• Both patients had successful outcomes for the unborn babies following delivery. By prioritizing the safety of both the mother and the child, tailored breast cancer treatments were given to the two individuals, leading to effective therapy.
What is the implication, and what should change now?
• Clinicians should increase their awareness and knowledge of PABC to avoid misdiagnosis or overlooking diagnoses.
Introduction
As a rare form of breast cancer, pregnancy-associated breast cancer (PABC) is infrequent, occurring in about 1 in 10,000 to 1 in 3,000 pregnant women, making up approximately 0.2% to 3.8% of all breast cancer cases (1-3). With the adoption of China’s three-child policy and the increasing age of mothers during childbirth, the incidence of PABC is expected to rise gradually. PABC presents unique challenges as it develops during pregnancy, requiring simultaneous consideration of both the mother’s treatment outcomes and the safety of the fetus. The diagnosis and treatment of PABC can be difficult due to fetal concerns, which can affect the overall prognosis (4,5). Since conducting randomized clinical trials poses ethical challenges, data on PABC mainly come from case reports or case-control studies. Consequently, controversies persist regarding the diagnosis and treatment of PABC at different stages (6,7). This article shares the diagnostic and treatment experiences of two cases of PABC, along with a comprehensive review of the existing literature, to summarize key points for diagnosing and treating PABC. This information provides valuable insights for breast surgeons in their clinical practice. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-223/rc).
Case presentation
Patient A
A 41-year-old woman at 30 weeks and 2 days of pregnancy was admitted due to a lump in her left breast that she had noticed for over a year. Due to busy work and neglect of physical examination, she did not seek medical treatment in advance. She was previously diagnosed with gestational diabetes mellitus in May 2022. Her obstetric history included 5 pregnancies with 2 deliveries (1 natural birth and 1 cesarean section) and 2 induced abortions. There was no family history of breast cancer.
Admission physical examination
Both breasts appear symmetrical, with nipples at the same level and no nipple retraction. No “orange peel sign” or “dimple sign” were observed. A palpable mass measuring about 3.0 cm × 2.0 cm was found in the 11 o’clock position of the left breast. The mass was firm, not easily movable, tender, and had unclear borders with the surrounding tissues. It was not attached to the chest wall or skin. No significant abnormalities were detected in the right breast. One enlarged lymph node was felt in the left armpit, while no enlarged lymph nodes were detected in the right armpit or along the clavicle (refer to Figures 1,2).
Breast ultrasonography (August 16, 2022)
On the left breast, approximately at 11 o’clock position, located about 15 mm from the nipple, a hypoechoic nodule measuring about 31 mm × 21 mm is identified. The borders are unclear, with heterogeneous internal echoes, and bright spots are visible. Multiple solid lymph nodes were found in the bilateral axillae. The larger node on the left was about 24 mm × 14 mm. Blood flow signals were visible in the lymph nodes. The boundary between the cortex and medulla was unclear. Calcification was visible inside the nodes. The larger lymph node on the right was about 13 mm × 8 mm (refer to Figures 3,4).


Obstetric ultrasonography (September 22, 2022)
The pregnancy was at an advanced stage, with a single fetus in a cephalic presentation. The fetus exhibited growth equivalent to 34 weeks and 3 days of gestation (refer to Figure 5). Electrocardiogram, abdominal ultrasound, thoracoabdominal computed tomography (CT), and cranial magnetic resonance imaging (MRI) with contrast performed after delivery revealed no notable abnormalities.

Biopsy results
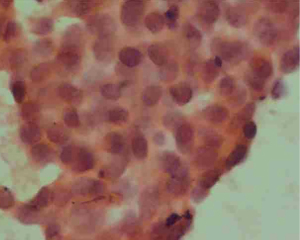
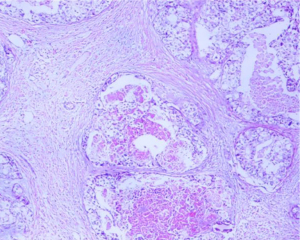
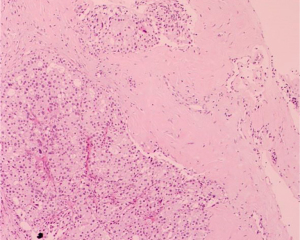
Left breast core needle biopsy revealed infiltrating carcinoma of a non-specific type, Grade 2. E-cadherin (+), P120 (membrane +), CK7 (+), estrogen receptor (ER) (0), progesterone receptor (PR) (0), human epidermal growth factor receptor 2 (HER2) (3+), Ki-67 (50%+), S100 (−), while P63, SMMHC, CK5/6 exhibited loss of the epithelium around the carcinoma nest (refer to Figure 6). Lymph node aspiration from the left armpit indicated malignant tumor cells, suggesting metastatic carcinoma (refer to Figure 7). The diagnosis was left-sided invasive carcinoma, classified as cT2N1M0, stage IIB, with HER2 overexpression subtype.
Treatment process
At 31 weeks and 1 day of pregnancy, the patient underwent one cycle of AC chemotherapy (consisting of cyclophosphamide 1 g, 600 mg/m2 and epirubicin 160 mg, 100 mg/m2). She delivered a baby girl by cesarean section at 35 weeks and 5 days of gestation, as shown in Figure 8. Following childbirth, starting on October 20, 2022, the patient underwent 5 cycles of neoadjuvant therapy with the THP regimen [comprising albumin-bound paclitaxel 400 mg (260 mg/m2), trastuzumab 600 mg (the first dose is 8 mg/kg, and the subsequent dose is 6 mg/kg), and pertuzumab 840 mg (the first dose is 840 mg, and the subsequent dose is 420 mg)] at 3-week intervals. On March 8, 2023, she underwent left breast-conserving surgery and left axillary lymph node dissection. Intraoperative frozen section analysis indicated a complete removal of the breast cancer post-neoadjuvant therapy on the left, with no detectable remaining tumor in the tumor bed. Furthermore, there was no tumor involvement observed in the upper, lower, inner, outer, or basal margins, nor in the skin. Paraffin section results also confirmed the absence of residual tumor within the tumor bed and margins. Lymph nodes examined from the left armpit showed no cancer metastasis (0/19), as depicted in Figures 9,10. Following the completion of the 6th cycle of THP chemotherapy after the surgery, the patient continued with targeted therapy [both trastuzumab 450 mg (6 mg/kg) and pertuzumab 420 mg, 11 cycles] and radiotherapy.



Patient B
A 33-year-old woman presented at 27 weeks and 1 day of pregnancy with a left breast lump that she had noticed for more than a month. There was no known family history of significant medical conditions. Her obstetric history included 5 pregnancies with 2 cesarean section deliveries and 2 miscarriages. There was no family history of breast cancer.
Admission physical examination
Both breasts appear symmetrical, with nipples at the same level and no nipple retraction. No “orange peel sign” or “dimple sign” were observed. A palpable mass measuring approximately 1.5 cm × 1.0 cm was identified in the 8 o’clock position of the left breast. The mass was firm, not easily movable, tender and had unclear borders with the surrounding tissues. It was not attached to the chest wall or skin. No notable abnormalities were found in the right breast. There were no enlarged lymph nodes felt in the armpits or along the clavicle (refer to Figures 11,12).
Breast ultrasonography (September 15, 2022)
The left breast showed thickened glandular tissue with disrupted echogenicity around the 8 o’clock position. Irregular hypoechoic areas were intermittently present, along with localized ductal dilation measuring about 4 mm at its widest point. Enhanced soft tissue echogenicity was observed around the periphery. No significantly enlarged lymph nodes were detected in the armpits bilaterally (refer to Figure 13).

Obstetric ultrasonography (September 28, 2022)
The pregnancy was in the mid-stage, with a singleton fetus in a cephalic presentation. The fetus exhibited a size equivalent to 27 weeks and 3 days of gestation (see Figure 14).

Biopsy results
A biopsy of the left breast lump revealed intraductal carcinoma (intermediate grade, with localized comedo necrosis). CD10, CK5/6, SMA demonstrated the presence of myoepithelial cells, ER (weakly positive, 30%), PR (weakly positive, 20%), HER2 (negative), Ki-67 (5%) (see Figure 15). Electrocardiogram and cardiac ultrasonography showed no abnormalities. No breast MRI, abdominal ultrasound, thoracoabdominal CT, or cranial MRI were performed. The diagnosis was left-sided intraductal carcinoma cTisN0M0, stage I, Luminal A subtype.

Treatment process
The patient underwent a left total mastectomy with left level I axillary lymph node dissection. Intraoperative frozen section pathology revealed that none of the 15 lymph nodes in sentinel lymph node exhibited tumor metastasis. Post-surgery paraffin section pathology indicated no tumor metastasis in the sentinel lymph node (0/15) and reported a case of intermediate-grade intraductal carcinoma in the left breast, accompanied by localized non-special type invasive carcinoma (with a maximum infiltrative diameter of approximately 1.1 mm). Intraductal carcinoma was observed in the nipple area, while tumor involvement was not observed in the upper or lower edges or basal margins.
Immunohistochemistry results for surgical specimen (invasive cancer part)
ER (weakly positive, 40%), HER2 (1+), Ki-67 (30%+), P53 (sporadic +), PR (weakly positive, 10%), and presence of myoepithelial cells (calponin, CK5/6, p63). Additional biopsies showed presence of myoepithelial cells (calponin, CK5/6, p63). Biopsy showed the presence of myoepithelial cells, albeit with partial loss of epithelium around the tumor nest. In the localized infiltrating carcinoma, E-cadherin was positive, ER was negative, HER2 was negative, Ki-67 was 40%, and PR was negative (see Figures 16,17). At 36 weeks of pregnancy, the patient delivered a male infant by cesarean section without any complications (refer to Figure 18). After giving birth, starting on December 21, 2022, she underwent 4 cycles of chemotherapy with the AC regimen (comprising cyclophosphamide 0.8 g, 600 mg/m2 and liposomal doxorubicin 50 mg, 35 mg/m2) and 4 cycles of chemotherapy with the T regimen (taxol 140 mg, 100 mg/m2) at 3-week intervals. Following the completion of chemotherapy, a breast MRI was performed, which indicated the absence of the left breast after excision. No masses or nodules were observed in the chest wall, and there were no abnormal enhancing areas after the administration of contrast. In the outer region of the right breast, a localized enhancing area measuring approximately 20 cm × 7 cm × 11 cm was visible. The time-signal curve indicated a Type II curve, and the apparent diffusion effect (ADC) value was approximately 1.1×10−3 s/mm2. A few focal enhancing lesions were observed within the right breast. No enlarged lymph nodes were detected in the armpits bilaterally. The MRI report indicated post-surgery changes after the removal of the left breast, a localized enhancing area in the outer region of the right breast [Breast Imaging Reporting and Data System (BI-RADS) Category 0], and hyperplasia in the right breast (refer to Figure 19). Breast ultrasound revealed that, following the left breast mastectomy, no prominent echogenic masses were observed in the left chest wall. In the right breast, a hypoechoic nodule measuring approximately 9 cm × 5 mm was detected around the 9 o’clock position, situated about 20 mm from the nipple. The border of the nodule was clear, and internal echogenicity remained uniform. Color Doppler flow imaging did not detect any evident blood flow signals within the nodule. No significantly enlarged lymph nodes were palpable in the supraclavicular or armpit regions bilaterally. The hypoechoic nodule in the right breast was classified as BI-RADS Category 3 (refer to Figure 20). Subsequently, right breast mass mastectomy with vacuum assisted biopsy system was performed. Intraoperative frozen section analysis indicated ductal carcinoma in situ (DCIS) in the right breast. Post-surgery paraffin section pathology results revealed intermediate-grade intraductal papillary carcinoma in the right breast, with fragmented tumor nestsv making it challenging to determine the surrounding myoepithelial cell conditions. Immunohistochemistry results for biopsy revealed: E-cadherin (+), ER (moderately strong +, approximately 80%), HER2 (2+), Ki-67 (10%+), P53 (sporadic +), PR (weakly negative to moderately positive, approximately 40%), and presence of myoepithelial cells (CK5/6, p63, calponin). Biopsy revealed the presence of myoepithelial cells around the majority of tumor nests, with fragmented tumor nests making it difficult to determine the surrounding myoepithelial cell conditions. Subsequent surgery included a right total mastectomy and sentinel lymph node biopsy. Intraoperative frozen section analysis indicated no tumor metastasis in the right sentinel lymph nodes (0/8). The post-surgery pathology of the right breast mastectomy specimen indicated no tumor residuals, with no tumor involvement in the nipple, basal margins, or upper and lower edges. The post-surgery pathology of the sentinel lymph nodes revealed no tumor metastasis (0/8) in the right axilla (see Figures 21-24). Following the procedure, the patient was initiated on endocrinotherapy with tamoxifen citrate for a duration of 5 years.







All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The definition of PABC varies among different studies
Most definitions of PABC include breast cancer diagnosed during pregnancy, known as breast cancer in pregnancy, as well as breast cancer diagnosed within 1 year after delivery (1).
Epidemiology
According to the literature, approximately 1 in every 3,000 to 10,000 pregnant women is diagnosed with PABC each year, constituting 0.2–3.8% of all breast cancer cases (2,3).
Clinical and pathological characteristics
Compared to non-PABC, PABC patients are not only younger but also present with more advanced T staging, a higher incidence of axillary lymph node metastasis, reduced expression of ER and PR, elevated expression of HER2, and greater invasiveness. These clinical and pathological characteristics often involve the lymphatic and vascular systems (8). Hormonal fluctuations during pregnancy lead to physiological breast changes, such as congestion, enlargement, nipple discharge, and increased breast tissue thickness, potentially leading to delayed patient presentation (3,9).
Diagnosis
Hormonal fluctuations during pregnancy and lactation induce physiological breast changes, including congestion, swelling, and increased glandular density. These alterations may hinder the early detection of breast lesions by clinical practitioners. Moreover, concerns about radiation exposure from imaging tests and contrast agents affecting fetal development pose challenges to the early diagnosis of PABC and heighten the risk of metastasis (10). Consequently, clinicians must emphasize every step of the diagnostic process. Breast surgeons should recognize the importance of screening for PABC when managing breast-related issues in pregnant and lactating patients. Thorough patient history taking is crucial, encompassing information about the onset of breast abnormalities, accompanying symptoms, pre-pregnancy examinations, and family history. Comprehensive clinical physical examinations should also be conducted. During physical examinations of pregnant women, increased vigilance is necessary when encountering breast masses, thickening, retraction, fixation, or suspiciously enlarged lymph nodes.
The careful selection of clinical imaging methods is crucial. Ultrasound examination, which is radiation-free and highly repeatable, offers high sensitivity for detecting lesions within dense breasts, particularly in young Chinese women. Breast ultrasound is recommended as the primary imaging method for evaluating breast and axillary lymph node conditions in pregnant and lactating women.
Breast ultrasound
Currently, breast ultrasound is given priority for assessing breast and axillary lymph node conditions in PABC patients. It does not involve radiation, has high repeatability, and is particularly sensitive for detecting lesions within dense breasts among Chinese women.
Mammography
The radiation dose of a mammography examination is approximately 0.004 Gy. When shielding the abdomen, fetal radiation exposure can be reduced by 50% to 75%, well below the threshold of 0.1 Gy for teratogenic radiation exposure (11). Therefore, in necessary situations, choosing mammography during pregnancy is not contraindicated.
Breast MRI
Routine breast MRI without contrast has limited diagnostic value for breast cancer. Dynamic contrast-enhanced breast MRI using gadolinium-based contrast agents can cross the placental barrier and have potential teratogenic effects. Thus, dynamic contrast-enhanced MRI is not recommended for pregnant women. However, contrast-enhanced MRI is relatively safe for lactating women, as recognized by the American College of Radiology (12).
Histopathological evaluation
For pregnant and lactating women, if imaging assessment of a breast lesion falls into BI-RADS 4–5 categories, core needle biopsy is recommended to obtain a histopathological diagnosis. Given the unique breast structure during pregnancy and lactation, individuals with BI-RADS 3 and a family history of breast cancer or other high-risk factors should be approached with caution. Close observation or consideration of biopsy is suggested (13).
Whole-body evaluation
Currently, there is no international consensus on whether lead shielding can reduce fetal radiation exposure to safe levels during development. Therefore, for PABC patients, it is recommended to prioritize abdominal ultrasound and chest X-ray (with abdominal shielding) for whole-body assessment. Chest and abdominal CT scans should be used cautiously (14). Whole-body bone scans [emission computed tomography (ECT)] and positron emission tomography (PET)-CT are nuclear medicine examinations that are contraindicated during pregnancy. Due to limitations in imaging options for pregnant patients, they should be informed about the possibility of an incomplete assessment of metastatic lesions.
Termination of pregnancy indications
For PABC patients with a gestational age of less than 13 weeks, it is recommended to consider terminating the pregnancy. Study (15) has shown that terminating the pregnancy does not improve the prognosis of PABC patients with a gestational age of 13 weeks or more. Compared to assessing and treating the disease postpartum, any effective treatment received during pregnancy can benefit PABC patients [overall survival rate: treatment during pregnancy (78.6%) vs. not receiving treatment during pregnancy (44.7%)] (15), as long as the safety of both the mother and the fetus is ensured. For PABC patients with a gestational age between 13 and 34 weeks, surgical and chemotherapy interventions can be considered based on the disease condition. The indications for terminating the pregnancy can follow those for normal pregnant women, and delivery is preferably planned after the 35th week of gestation, allowing for either natural delivery or cesarean section. In cases requiring preterm delivery, efforts should be made to promote fetal lung maturation. For PABC patients with a gestational age of 35 weeks or more, it is advisable to complete breast cancer assessment and initiate standard treatment after delivery.
The potential impact of anti-cancer drugs and radiation on the developing fetus must be carefully considered. Before deciding whether to continue the pregnancy, a detailed analysis of the potential benefits and risks of the treatment regimen is essential. Patients should be fully informed about the potential risks associated with receiving anti-tumor treatment during pregnancy, and their preferences should be respected.
Treatment
The principles of treating breastfeeding-related breast cancer are similar to those for non-PABC cases. PABC treatment should consider both the therapeutic efficacy for pregnant breast cancer patients and the safety of the developing fetus.
Surgical treatment
The surgical principles for early-stage PABC patients are similar to those for non-PABC patients. For early-stage PABC patients eligible for R0 resection, breast surgeons should adopt a multidisciplinary treatment (MDT) approach, collaborating with obstetrics, anesthesiology, and nursing departments to collectively assess the health of the pregnant woman and the fetus and prepare for the perioperative period.
Timing of surgery selection
PABC surgery does not increase the risk of maternal mortality or fetal congenital defects, and delaying surgery may increase the potential risk of breast cancer metastasis (10). Research indicates that during early pregnancy (gestational age less than 13 weeks), breast surgery is associated with a higher risk of spontaneous abortion or reduced birth weight of the fetus. Conversely, performing surgery in late pregnancy (gestational age 28 weeks or more) may lead to premature birth. Surgery during mid-pregnancy (gestational age 13–27 weeks) is relatively safer (12).
Surgical approach selection
While adhering to the principle of achieving an R0 resection, clinical practitioners should choose an appropriate surgical approach based on the patient’s clinical staging.
Modified radical mastectomy: this is the standard approach for early-stage PABC patients with axillary lymph node metastasis. The internationally used isosulfan blue and the domestically applied methylene blue dye may induce allergic reactions in the mother, and their safety in pregnant women remains to be conclusively established (16).
Breast-conserving surgery: research (11) indicates that the survival rates of stage I and II PABC patients are similar between breast-conserving surgery and mastectomy. Due to the clear teratogenic risk of radiation therapy to the fetus, prioritizing breast-conserving surgery for PABC patients is generally not recommended. However, since the safety of administering chemotherapy to PABC patients during mid to late pregnancy (gestational age ≥13 weeks) has gained recognition, a comprehensive treatment plan involving 4–6 cycles of chemotherapy post breast-conserving surgery, with radiotherapy deferred until postpartum, makes breast-conserving surgery a viable option for some PABC patients (10). It must be emphasized that due to changes in breast appearance during pregnancy and the reasons for postpartum breast restitution, post-breast-conserving surgery aesthetic changes may occur.
Immediate reconstruction surgery: given the alterations in breast appearance following pregnancy and lactation, immediate breast reconstruction is not recommended for PABC patients.
Systemic treatment
Chemotherapy
Chemotherapy during pregnancy can lead to complications such as gestational hypertension, intrauterine growth restriction, reduced fetal birth weight, and premature birth (13). Particularly, chemotherapy in early pregnancy may result in premature birth and fetal deformities. Pregnant PABC patients requiring chemotherapy should receive it during mid to late pregnancy (gestational age ≥13 weeks). It is advisable to consult with obstetricians within a MDT to refine fetal malformation screening (17,18), collectively assess and plan the chemotherapy regimen, and determine the optimal delivery timing. Vigilant monitoring of the mother and evaluation of fetal developmental indicators should be maintained during chemotherapy, with prompt management of any abnormalities. Chemotherapy may induce bone marrow suppression, potentially increasing the risk of infection and bleeding for both the mother and the newborn after delivery. Therefore, chemotherapy should be temporarily paused by gestational week 34 in preparation for childbirth. Additionally, considering the naturally elevated white blood cell count in pregnant women, the possibility of underlying bone marrow suppression should be considered. Chemotherapy dosages should be calculated based on actual body surface area, and dense dosing regimens are not recommended (19).
Anthracycline drugs can be widely used, while taxanes are approved by the European Society of Medical Oncology (ESMO) guidelines. However, cyclophosphamide carries a 1% teratogenic risk when used during mid to late pregnancy. Platinum-based agents can cross the human blood-placental barrier, posing risks of restricted fetal growth and malformation (3,9,12,17).
Endocrine therapy
Endocrine therapy can result in fetal malformations, vaginal bleeding, and miscarriage (12,20). It is not advisable to administer endocrine therapy to PABC patients during pregnancy, and it should be postponed until after childbirth.
Targeted therapy
The HER2 pathway is involved in early conception and implantation processes, playing a critical role in fetal organ development. Research (20) has shown HER2 expression in placental and fetal kidney tissues during late-stage pregnancy, and trastuzumab’s toxicity on fetal kidney cells can lead to reduced amniotic fluid. Pertuzumab and tyrosine kinase inhibitors have not been studied in pregnant women. Therefore, it is recommended to delay the use of targeted therapy drugs such as trastuzumab until after delivery.
Radiotherapy
The standard radiotherapy dose is 50 Gy, significantly exceeding the threshold of 100 mGy for adverse effects on pregnant women. Therefore, the use of radiotherapy during pregnancy is not recommended (21).
Conclusions
Patient A presented with invasive breast cancer and axillary lymph node metastasis, confirmed through ultrasound and core needle biopsy. Initial tests, including electrocardiography, abdominal ultrasound, and cranial MRI, revealed no significant abnormalities. A comprehensive assessment led to a diagnosis of left-sided invasive breast cancer, categorized as cT2N1M0, HER2-positive subtype, and classified as stage IIB. According to the guidelines, neoadjuvant chemotherapy was recommended to shrink the tumor and downstage the disease. However, at 31 weeks of gestation, with only one month left until week 35, the patient faced a choice between two treatment options. The first option involved an immediate cesarean section followed by guideline-based treatment. However, this option carried a higher risk of premature birth, along with increased treatment expenses. The second option was to continue the pregnancy until week 35, undergoing neoadjuvant chemotherapy primarily with anthracyclines and cyclophosphamide, which posed lesser harm to the fetus. Following improved fetal maturation, delivery would be scheduled, followed by neoadjuvant therapy using the THP regimen, surgery, and radiotherapy. This alternative raised the risk of adverse intrauterine consequences. A multidisciplinary discussion, along with adherence to the patient’s preferences, led to the decision to implement the following treatment sequence: chemotherapy, childbirth, neoadjuvant therapy, surgery, and radiotherapy. Given the patient’s pregnancy and HER2-positive subtype, a cautious approach was taken. Despite the HER2-positive classification, the safer AC regimen was chosen for the initial chemotherapy. Encouragingly, a follow-up ultrasound after the first chemotherapy cycle indicated no disease progression and a healthy fetal status. The patient successfully delivered around week 35. After childbirth, neoadjuvant therapy was resumed, revealing substantial tumor progression in the 50 days following the initial chemotherapy, with a favorable fetal condition. A total of 5 cycles of the THP regimen (3-week intervals) were administered, followed by breast-conserving surgery. Post-surgery pathology indicated a complete pathologic response. Subsequent to surgery, radiotherapy and targeted therapy were administered as per guidelines. Patient B was diagnosed with an early-stage DCIS at 27 weeks of pregnancy. The primary goal of the initial treatment for DCIS is to prevent its progression into invasive cancer (18). Despite a comprehensive biopsy, the potential for unbiopsied infiltrating components remained. Additionally, the patient reported an increase in the size of the lump. Consequently, after extensive multidisciplinary discussions, an initial surgical treatment plan was formulated. In selecting the surgical approach, the tumor stage and imaging data were taken into account. While breast-conserving surgery was considered, a physical examination revealed significant enlargement and thickening of the left breast. Therefore, the decision was made to proceed with unilateral mastectomy. According to guidelines, sentinel lymph node biopsy is recommended for axillary lymph nodes, with the option of axillary lymph node dissection based on the results. However, due to concerns about the safety of tracers during pregnancy, a direct axillary lymph node dissection strategy was adopted to minimize the risk of residual lymph node involvement and potential recurrence. Following comprehensive preoperative preparations, the patient underwent planned left breast mastectomy along with clearance of level I axillary lymph nodes. Postoperative pathology indicated infiltration and a high Ki-67 value of 40% [Ki-67 cutoff value set at 20%, with values >20% indicating high expression (19)], indicative of increased invasiveness. After further multidisciplinary discussions, a strategy was developed that combined childbirth and postoperative adjuvant chemotherapy to maximize benefits for both the patient and the fetus. Subsequently, the patient successfully underwent childbirth and received four rounds of postpartum adjuvant chemotherapy as scheduled. Following the completion of chemotherapy, a new lump was detected in the right breast, possibly due to elevated estrogen levels during pregnancy. Excision of the lump revealed DCIS. Consequently, a right breast mastectomy accompanied by sentinel lymph node biopsy was performed. Post-surgery pathology results indicated no tumor involvement in the sentinel lymph nodes. Post-surgery, the patient continued with endocrine therapy as planned. Both patients delivered healthy babies with favorable outcomes, showing no apparent anomalies or abnormalities. Patient A achieved complete pathological remission through treatment, while patient B, despite developing a new lump in the contralateral breast within 6 months post-surgery, had the lump excised and confirmed to be DCIS through postoperative pathology assessment. Patient B is currently undergoing combined treatment with endocrine therapy. For these two PABC patients, personalized treatments were tailored based on their distinct clinical characteristics. This approach effectively prevented the progression of breast cancer during pregnancy while ensuring the safety of the fetuses. This underscores the importance of proactive and effective personalized treatments for PABC patients, which can lead to benefits for both the mother and the child. Clinical practitioners should also enhance their awareness and understanding of PABC patients to avoid missed or incorrect diagnoses.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-223/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-223/prf
Funding: The present work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-223/coif). Yuanliang Wang reports the funding from the Doctoral Research Start-up Foundation of Zunyi Medical University [No. Yuanzi (2022) No. 12] and the Science and Technology Plan Project Foundation of Zunyi city [No. Zunshi Kehe HZ Zi (2022) No. 262]. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amant F, Loibl S, Neven P, et al. Breast cancer in pregnancy. Lancet 2012;379:570-9. [Crossref] [PubMed]
- McGrath SE, Ring A. Chemotherapy for breast cancer in pregnancy: evidence and guidance for oncologists. Ther Adv Med Oncol 2011;3:73-83. [Crossref] [PubMed]
- Boere I, Lok C, Poortmans P, et al. Breast cancer during pregnancy: epidemiology, phenotypes, presentation during pregnancy and therapeutic modalities. Best Pract Res Clin Obstet Gynaecol 2022;82:46-59. [Crossref] [PubMed]
- Han BY, Li XG, Zhao HY, et al. Clinical features and survival of pregnancy-associated breast cancer: a retrospective study of 203 cases in China. BMC Cancer 2020;20:244. [Crossref] [PubMed]
- O'Sullivan CC, Irshad S, Wang Z, et al. Clinico-pathologic features, treatment and outcomes of breast cancer during pregnancy or the post-partum period. Breast Cancer Res Treat 2020;180:695-706. [Crossref] [PubMed]
- Albach A, Dixon LB 3rd, Sadruddin S, et al. Diagnosis and Management of Metastatic Breast Cancer in a 33-year-old Pregnant Female: A Case Report. Cureus 2019;11:e5240. [Crossref] [PubMed]
- Untanas A, Grigaitė I, Briedienė R. Imaging in pregnancy-associated breast cancer: a case report. Acta Med Litu 2019;26:134-9. [Crossref] [PubMed]
- Suleman K, Osmani AH, Al Hashem H, et al. Behavior and Outcomes of Pregnancy Associated Breast Cancer. Asian Pac J Cancer Prev 2019;20:135-8. [Crossref] [PubMed]
- Yang WT, Dryden MJ, Gwyn K, et al. Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology 2006;239:52-60. [Crossref] [PubMed]
- Viswanathan S, Ramaswamy B. Pregnancy-associated breast cancer. Clin Obstet Gynecol 2011;54:546-55. [Crossref] [PubMed]
- Cordeiro CN, Gemignani ML. Breast Cancer in Pregnancy: Avoiding Fetal Harm When Maternal Treatment Is Necessary. Breast J 2017;23:200-5. [Crossref] [PubMed]
- Shachar SS, Gallagher K, McGuire K, et al. Multidisciplinary Management of Breast Cancer During Pregnancy. Oncologist 2017;22:324-34. [Crossref] [PubMed]
- Loibl S, Schmidt A, Gentilini O, et al. Breast Cancer Diagnosed During Pregnancy: Adapting Recent Advances in Breast Cancer Care for Pregnant Patients. JAMA Oncol 2015;1:1145-53. [Crossref] [PubMed]
- Woussen S, Lopez-Rendon X, Vanbeckevoort D, et al. Clinical indications and radiation doses to the conceptus associated with CT imaging in pregnancy: a retrospective study. Eur Radiol 2016;26:979-85. [Crossref] [PubMed]
- Yu HH, Cheung PS, Leung RC, et al. Current management of pregnancy-associated breast cancer. Hong Kong Med J 2017;23:387-94. [PubMed]
- Cardonick E. Pregnancy-associated breast cancer: optimal treatment options. Int J Womens Health 2014;6:935-43. [Crossref] [PubMed]
- Peccatori FA, Azim HA Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi160-70. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Blair SL, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw 2014;12:542-90. [Crossref] [PubMed]
- Azim HA Jr, Del Mastro L, Scarfone G, et al. Treatment of breast cancer during pregnancy: regimen selection, pregnancy monitoring and more. Breast 2011;20:1-6. [Crossref] [PubMed]
- Goller SS, Markert UR, Fröhlich K. Trastuzumab in the Treatment of Pregnant Breast Cancer Patients - an Overview of the Literature. Geburtshilfe Frauenheilkd 2019;79:618-25. [Crossref] [PubMed]
- Ray JG, Vermeulen MJ, Bharatha A, et al. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016;316:952-61. [Crossref] [PubMed]
Cite this article as: Wang Y, Wang Y, Sun S. Individualized treatment of pregnancy-associated breast cancer: a report of two cases and literature review. AME Case Rep 2025;9:69.







