
Robot-assisted partial gastrectomy with Billroth I anastomosis for gastric plexiform fibromyxoma in children: the first case report and literature review
Highlight box
Key findings
• The da Vinci robotic system is safe and feasible to perform partial gastrectomy with Billroth I anastomosis for pediatric gastric plexiform fibromyxoma (PF), with good short-term postoperative outcomes.
What is known and what is new?
• Pediatric PF is rare, and there are no reports on the treatment of pediatric PF with robotic surgical systems for the time being.
• Robotic surgical system is safe and feasible for pediatric gastric PF.
What is the implication, and what should change now?
• Robot-assisted surgery can be an effective tool for treating pediatric gastric PF.
Introduction
Plexiform fibromyxoma (PF) is an extremely rare mesenchymal tumor, which was first reported by Takahashi et al. in 2007 (1) and named by the World Health Organization (WHO) in 2010 in the classification of digestive system tumors. To date, only 123 cases have been reported worldwide (2), which mainly occur in adults and occasionally in children and adolescents. As a result, there are only 13 reported cases of PF in children worldwide, and no robotic surgery has been reported. On October 20, 2023, we successfully performed a robot-assisted partial gastrectomy with Billroth I anastomosis to treat gastric PF and the child has recovered well. To our knowledge, no similar cases have been reported in the literature, which suggests that this may represent the first documented case worldwide. We reviewed relevant literature and summarized the experience of robot-assisted partial gastrectomy with Billroth I anastomosis in the treatment of PF surgery for children. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-214/rc).
Case presentation
A 7-year-old Chinese female patient, with a weight of 22 kg, was admitted to Guangzhou Women and Children’s Medical Center, due to “having pallor for 4 months and having a tumor in the gastric antrum for over 2 weeks”. Four months ago, the child developed paleness, dizziness and fatigue, without any apparent cause. She visited a local hospital for a check-up, which revealed a hemoglobin (Hb) level of 57 g/L and positive stool occult blood. The enhancement CT found a mass in her gastric antrum, approximately 4.0 cm × 3.5 cm × 3.0 cm in size with the nature to be determined. The electronic gastroscopy revealed a mass in the gastric antrum and pylorus orifice, with a smooth surface, and its base was located on the lesser curvature side and pylorus. After consultation by the multi-disciplinary treatment (MDT), we decided to perform robot-assisted partial gastrectomy with Billroth I anastomosis for her to remove the mass. All procedures performed in this case were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Surgical instruments and surgical trocar layout
The surgery was performed using the da Vinci Xi robotic surgery system, surgical instruments including: fenestrated bipolar forceps, monopolar curved scissors (hot shears), and Large SutureCut Needle Driver. The child received general anesthesia via endotracheal intubation and then took a supine position with the entire body elevated by 5 cm. During the surgery, R2 was the No. 2 robotic arm (fenestrated bipolar forceps), R3 was the No. 3 robotic arm (observation port), R4 was the No. 4 robotic arm (monopolar curved scissors), and A was the auxiliary port located in the lower left abdomen (as shown in Figure 1).

Surgical procedures
During the surgery, we suspended the ligamentum teres hepatis to fully expose the gastric antrum and pylorus, then we found the mass was located in the gastric antrum and the lesser curvature of the stomach, which involved the anterior wall of the pylorus, firm in texture, pale in color, and 4.0 cm × 3.5 cm × 3.0 cm in size approximately. What’s more, we also found that part of the mass had broken through the serosal layer (as shown in Figure 2). The boundary between the tumor and the surrounding tissue was still clear. No evidence of abdominal cavity metastasis or enlargement of sentinel lymph nodes of the lesser and greater curvatures of the stomach was observed. We took a lymph node at the pylorus for intraoperative frozen biopsy, which reported indicated reactive enlargement and no malignant components.
After the relationship between the mass and the prepyloric vein, right gastric omental artery, extrahepatic biliary tract, pancreas, splenic artery, etc. was identified (as shown in Figure 3), we used the monopolar curved scissors to mark the boundary of the mass, and then cut 2 cm along the edge of the mass, about 1 cm in layers. We found the mass has not broken through the mucosal layer during surgery (as shown in Figure 4). After draining the contents of the stomach and duodenum, we resected the mass stratified and completely (as shown in Figures 5,6), and less blood loss during resection. Then we put the specimen into a specimen bag, rinsed and explored the surgical operative field to make sure no collateral damage (as shown in Figure 7). Then we found the pylorus only had about 1/4 of the posterior wall remaining. We used the 4-0 barbed suture to anastomose the stomach and duodenum (Billroth I anastomosis), injected 60 mL of methylene blue solution and 250 mL of air through the gastric tube, so the stomach was well filled, and the methylene blue solution and gas passed smoothly through the duodenum and entered the jejunum. No leakage was found at the anastomotic site (as shown in Figure 8). Then we explored the enterocoelia to make sure no collateral damage occurred, removed the robotic arm, removed the specimen through the umbilical incision for examination (as shown in Figure 9), closed the abdominal incision one by one and finished the surgery.



The surgery took a total of 145 minutes, with 15 minutes for the preparation of the da Vinci robotic system and 120 minutes for the robot assisted partial gastrectomy with Billroth I anastomosis. The blood loss was 10 mL and 0.5 U of red blood cell suspension was infused.
Postoperative situation
On the 3rd day after surgery, the patient resumed anal venting and defecation, and began to drink water. On the 5th day after the gastric tube was clamped, there was no nausea or vomiting. Then we removed the gastric tube, gave the child patient who had no discomfort a liquid diet, and gave her normal diet on the 7th day after surgery. On the 10th day after surgery, after all indicators were rechecked and showed no abnormalities, the child was discharged.
Pathological diagnosis
Under the microscope, the tumor appears clustered and multinodular, infiltrating from the mucosa to the muscular layer and locally penetrating the serosal layer. The tumor cells are oval and short fusiform, with pale cytoplasm and rare nuclear division. The stroma shows mucinous and fibromucinous changes, and abundant blood vessels are observed (as shown in Figures 10,11). Immunohistochemistry: SMA (weak+), DOG1 (+), CD34 (−), CD117 (−).

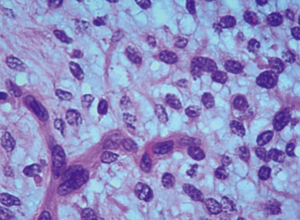
Pathological diagnosis: (I) (gastric tumor) plexiform fibromyxoid tumor; (II) pyloric lymph nodes: no tumor found. Genetic testing of the family: trios whole exome sequencing V6 (200×); high-throughput sequencing of the family: no clear pathogenic mutations related to the disease phenotype were found.
Followed-up after operation
After a three-month follow-up period, there were no abnormalities detected in the blood routine and biochemistry tests, no indications of mass recurrence in the abdominal CT scan, and no irregularities observed in the upper digestive tract angiography. Currently, the child has resumed a normal diet and is experiencing no acid reflux, belching, vomiting, black stool, bloody stool, or any other abnormal symptoms.
“Initially, I was apprehensive about the surgical procedure that involved robot-assisted surgery for my child, which had no report of robot-assisted partial gastrectomy with Billroth I anastomosis for gastric mass in children”, the child’s parents told us while we introduced the diagnosis, treatment plan and surgical plan for their child. “However, I grew more confident about letting my child undergo robot-assisted surgery after the doctors’ sufficient preparation before operation and a review of historical case studies (ensuring those cases’ privacy)”. In the end, the child’s parents were satisfied with the outcomes and told us they were grateful for the advanced technological capabilities and the expertise of the doctors who made this possible for their child.
Discussion
PF is a newly named disease in the 2010 classification of digestive system tumors by the WHO. It mainly occurs in the antrum of the stomach, but there are also reports of PF occurring in other places such as the torso (n=10, 8.3%), fundus of the eye (n=4, 3.3%) (1,2), esophagus (3), duodenum (4), jejunum (5,6), gallbladder (7), and colon (8). It is common in adults and occasionally occurs in children and adolescents.
The disease usually has no specific clinical symptoms, but it can lead to dull pain in the upper abdomen, hematemesis, melena, pyloric obstruction, etc. Previous research has indicated that postoperative follow-up periods for PF cases showed no instances of recurrence or metastasis. However, more recent studies have presented contrasting views. For instance, a case involving a 50-year-old female with PF recurrence and metastasis, which ultimately led to her demise, has been documented (9). Additionally, there are reports of vascular and visceral invasion associated with PF (4,10).
Consequently, the classification of PF as a benign or malignant lesion remains an area that requires further investigation. But one thing is certain: if the resection is incomplete, there is a possibility of recurrence. Complete surgical resection is the only method to radically cure PF (11-13).
Previous reports on PF surgical methods for the stomach include gastric mass resection with Braun anastomosis (12), laparoscopic gastric tumor resection (13), open partial gastrectomy (14), endoscopic submucosal dissection converted to subtotal gastrectomy (Billroth I) (15), radical distal gastrectomy with Billroth I anastomosis (2), partial distal gastrectomy with Billroth II anastomosis (16), endoscopic submucosal dissection (3), etc. We have reviewed the literature on pediatric PF and summarized it in Table 1. We found that there are no relevant reports on the robot-assisted surgery.
Table 1
| Author [year] | Number of cases | Sex (M/F) | Age (Y) | Mass location | Operation | Size (cm) |
|---|---|---|---|---|---|---|
| Goyal S [2023] (2) | 1 | M | 15 | Gastric antrum | Distal gastrectomy, Billroth I reconstruction | 7.2×5.1×4 |
| Duckworth LV [2014] (4) | 2 | F | 11 | Gastric pylorus | Laparoscopic distal gastrectomy | 3.5 (max diameter) |
| F | 16 | In the posterior mediastinum near the esophagus | Thoracoscopic mass resection | 3.2 (max diameter) | ||
| Bugeda Gómez P [2024] (6) | 1 | M | 0.25 | Jejunum | Mass and associated bowel resection | 1.6 (max diameter) |
| Miettinen M [2009] (10) | 2 | F | 7 | Antrum, pylorus, duodenal bulb | Excision of tumor gastric wall resection at the tumor attachment | 15 (max diameter) |
| F | 16 | Gastric antrum | Distal gastrectomy | 10 (max diameter) | ||
| Morris MW [2016] (11) | 2 | F | 9 | Gastric antrum | Laparotomy partial gastrectomy | 5 (max diameter) |
| F | 11 | Gastric antrum, duodenal bulb | Distal gastrectomy | 3.5 (max diameter) | ||
| Szurian K [2017] (12) | 1 | F | 16 | Gastric antrum | Distal gastrectomy, Billroth II reconstruction | 6.5 (max diameter) |
| Djurić Z [2019] (13) | 1 | M | 14 | Gastric antrum | Partial gastrectomy | 5 (max diameter) |
| Wang FH [2012] (14) | 1 | M | 12 | Gastric antrum | Partial gastrectomy and mass resection | 5×5×5 |
| Fukazawa M [2019] (15) | 1 | F | 14 | Gastric antrum | Partial gastrectomy | 5.5 (max diameter) |
| Li L [2016] (16) | 1 | M | 11 | Gastric pylorus | Partial gastrectomy and mass resection | 20×15×14 |
F, female; M, male; max, maximum; Y, years.
However, the aforementioned surgical methods are not fully suitable for this child. For instance, laparotomy gastric mass resection and digestive tract reconstruction may result in significant injury. Laparoscopic gastric mass resection and digestive tract reconstruction could be difficult to perform when the mass involves the posterior wall of the stomach because of its limited operation angles. Endoscopic piecemeal mucosal resection (EPMR) is likely to destroy the integrity of the tumor, making it difficult to ensure R0 resection. Based on this, we attempt to use a robotic surgical system to perform partial gastrectomy and gastrointestinal reconstruction.
Since there are no reports about the robot-assisted partial gastrectomy and gastrointestinal reconstruction in children, there is limited experience to refer to. Based on the recommendations in the “Chinese Guidelines for Robotic Gastric Cancer Surgery” and considering the characteristics of this child, our team made the following adjustments during the surgery: (I) position and puncture point: horizontal position, overall cushion height 5 cm. R3 is umbilical puncture which was used as an observation port; R2 and R4 are located at the umbilical horizontal line, 6 cm apart from R3. Assistant hole A is 3 cm below the midpoint of the line connecting R3 and R4, which can avoid interference between the various robotic arms and assistants effectively. (II) Enlarge the surgical field: We exposed the target surgical area adequately by suspending the round ligament of the liver. (III) Clarify the relationship of the mass and surrounding tissues: to determine whether there is mass invasive growth or intraperitoneal metastasis. (IV) When the malignancy of the mass cannot be determined: take lymph nodes near the mass for rapid biopsy. (V) Selection of surgical instruments: we found that the monopolar curved scissors are more advantageous than ultrasonic knife in subtle manipulation because of its multiple angles. (VI) Reduce wound bleeding: we found that stratified resection of the mass according to the structure of the gastric wall can reduce the wound bleeding. (VII) The residual anterior wall of the gastric antrum and pylorus are transversely anastomosed to avoid a significant difference in the diameter of the gastro-duodenal anastomosis. (VIII) After the anastomosis, we injected methylene blue solution and air through the gastric tube to ensure that the stomach-duodenum anastomosis is unobstructed and free of leakage. (IX) We also pay attention to the operational details of rapid rehabilitation such as not placing the abdominal drainage tube because of the reliable reconstruction.
Since 2007, robot-assisted gastrectomy has emerged as a new option for the treatment of gastric cancer (17). The da Vinci Xi robotic system boasts significant technical advantages over laparoscopic surgery, which fosters higher surgical expectations (18,19). A retrospective study of 2,084 cases showed that there was no statistically significant difference in recurrence-free survival and overall survival between laparoscopic and robot-assisted gastrectomy among patients at the same stage; the robot-assisted surgery resulted in less blood loss but took more operation time than the laparoscopic surgery (20). And the study by Koh et al. indicates that robot-assisted surgery can safely replace traditional surgery (21).
At present, robot-assisted surgery has been gradually carried out in pediatric surgery, but compared with adult surgery, the experience is still insufficient and the diseases are still limited. Robotic-surgical system is mainly used in pediatric general surgery for the treatment of Hirschsprung’s disease (such as robot-assisted transanal endorectal pull-through) and congenital biliary dilatation (such as robot-assisted cholecystectomy, common bile duct excision, and embedding hepaticojejunostomy) (22). In 2023, Jixiao Zeng’s team reported the first case of robot-assisted pyloric-preserving pancreaticoduodenectomy to remove rhabdomyosarcoma in duodenal ampulla of a 3-year-old boy and the first case of robotic hepatectomy without the first hepatic portal control to remove a 14-month-old child with hepatoblastoma. Those applications suggested the indication of robot-assisted surgery in pediatric could be further expanded, and the experience of robot-assisted surgery in adult cannot be directly applied (23,24).
Conclusions
In summary, this study confirms that the use of the da Vinci robotic system to perform partial gastrectomy with Billroth I anastomosis for pediatric gastric PF is safe and feasible, with good short-term postoperative outcomes. Currently, there are no relevant reports on the use of robot-assisted surgery for pediatric gastric PF, and its clinical efficacy still needs further verification.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-214/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-214/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-214/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takahashi Y, Shimizu S, Ishida T, et al. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol 2007;31:724-8. [Crossref] [PubMed]
- Goyal S, Beso P, Khullar R, et al. Plexiform fibromyxoma of the stomach: An underrecognized entity! Indian J Pathol Microbiol 2023;66:343-6. [Crossref] [PubMed]
- Su HA, Yen HH, Chen CJ. An Update on Clinicopathological and Molecular Features of Plexiform Fibromyxoma. Can J Gastroenterol Hepatol 2019;2019:3960920. [Crossref] [PubMed]
- Duckworth LV, Gonzalez RS, Martelli M, et al. Plexiform fibromyxoma: report of two pediatric cases and review of the literature. Pediatr Dev Pathol 2014;17:21-7. [Crossref] [PubMed]
- Yang W, Li L, Liu Y, et al. Duodenal plexiform angiomyxoid myofibroblastic tumor: One case report. Chinese Journal of Magnetic Resonance Imaging 2023;(12):109-110+126.
- Bugeda Gómez P, Costa-Roig A, Montecino Romanini C, et al. Pediatric Plexiform Fibromyxoma: A Case Report. J Pediatr Hematol Oncol 2024;46:e251-3. [Crossref] [PubMed]
- Spans L, Fletcher CD, Antonescu CR, et al. Recurrent MALAT1-GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J Pathol 2016;239:335-43. [Crossref] [PubMed]
- Fassan M, Salmaso R, Saraggi D, et al. Plexiform fibromyxoma of the gallbladder. Pathologica 2015;107:181-4. [PubMed]
- Ayyanar P, Nayak HK, Samal SC, et al. Recurrent plexiform angiomyxoid myofibroblastic tumour (PAMT) of the stomach with aggressive behaviour. Pathology 2022;54:650-4. [Crossref] [PubMed]
- Miettinen M, Makhlouf HR, Sobin LH, et al. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol 2009;33:1624-32. [Crossref] [PubMed]
- Morris MW, Sullivan L, Sawaya DE, et al. Gastric plexiform fibromyxoma tumor in a child – Case report and review of the literature. Journal of Pediatric Surgery Case Reports 2016;4:38-41. [Crossref]
- Szurian K, Till H, Amerstorfer E, et al. Rarity among benign gastric tumors: Plexiform fibromyxoma–- Report of two cases. World J Gastroenterol 2017;23:5817-22. [Crossref] [PubMed]
- Djurić Z, Stojšić Z, Radulović S, et al. Plexiform Fibromyxoma: A Rare Benign Gastric Tumor. J Pediatr Gastroenterol Nutr 2019;68:e67. [Crossref] [PubMed]
- Wang FH, Chen ZR, Niu HL, et al. Plexiform fibromyxoma of stomach: a distinctive benign tumor of gastric antrum. Zhonghua Bing Li Xue Za Zhi 2012;41:190-1. [PubMed]
- Fukazawa M, Koga H, Hiroshige S, et al. Pediatric plexiform fibromyxoma: A PRISMA-compliant systematic literature review. Medicine (Baltimore) 2019;98:e14186. [Crossref] [PubMed]
- Li L, Han C, Xu F. Plexiform fibromyxoma of stomach: a newly recognized mesenchymal tumor. Chinese Journal of Diagnostic Pathology 2016;23:825-827+832.
- Anderson C, Ellenhorn J, Hellan M, et al. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 2007;21:1662-6. [Crossref] [PubMed]
- Song J, Oh SJ, Kang WH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-32. [Crossref] [PubMed]
- Song J, Kang WH, Oh SJ, et al. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc 2009;23:1204-11. [Crossref] [PubMed]
- Shin HJ, Son SY, Wang B, et al. Long-term Comparison of Robotic and Laparoscopic Gastrectomy for Gastric Cancer: A Propensity Score-weighted Analysis of 2084 Consecutive Patients. Ann Surg 2021;274:128-37. [Crossref] [PubMed]
- Koh DH, Jang WS, Park JW, et al. Efficacy and Safety of Robotic Procedures Performed Using the da Vinci Robotic Surgical System at a Single Institute in Korea: Experience with 10000 Cases. Yonsei Med J 2018;59:975-81. [Crossref] [PubMed]
- Zhang S, Gao Z, Tou J, et al. Current applications of robotic procedures in pediatric surgery. Journal of Clinical Pediatric Surgery 2021;20:701-7.
- Xu X, Zeng J, Luo Y, et al. Robotic hepatectomy in a 14-month-old child of hepatoblastoma without the first hepatic portal control: a first case report in the world. Journal of Clinical Pediatric Surgery 2023;22:1114-8.
- Zeng J, Xu X, Liu F, et al. Robot-assisted pylorus-preserving pancreaticoduodenectomy for rhabdomyosarcoma in hepatopancreatic ampulla of a child: the first case report (with video). Chinese Journal of Robotic Surgery 2023;4:606-11.
Cite this article as: Ye Z, Zeng J, Lu H, Liu F, Xu X, Luo Y, Zhang H, Lan M, Tao B, Liang Z, Wen L. Robot-assisted partial gastrectomy with Billroth I anastomosis for gastric plexiform fibromyxoma in children: the first case report and literature review. AME Case Rep 2025;9:70.







