Drug desensitization in 17-year-old male with Mast Cell Activation Syndrome, pneumonia, and antibiotic hypersensitivities
Introduction
The development of drug desensitization, also known as temporary induction of drug tolerance, is designed to provide essential medications for patients with drug allergy. Temporary tolerance induction can be used in IgE-mediated reactions that include anaphylaxis, and non-IgE mediated reactions (1). Type I hypersensitivity reactions, including anaphylaxis, result from the release of mediators such as histamine, serine proteases, heparin, prostaglandins, and cytokines from IgE-sensitized mast cells, which requires prior exposure for sensitization (1,2). In comparison, non-IgE mediated immediate (pseudo-allergic or “anaphylactoid”) reactions are characterized by the activation of mast cell and mediator release without involvement of the IgE-mediated signaling (3). Both reactions, regardless of etiology, may lead to local tissue edema, vascular leak, shock and possible mortality (1,3).
Desensitization is the act of providing successively increasing doses until the full therapeutic dose is achieved, enabling basophil and mast cell stabilization. The mechanism is not currently fully understood but is thought to be due to subthreshold antigen stimulation by hapten-carrier conjugates. Normal protocols consist of administration of progressive doses of a drug every 15 to 30 minutes until a full therapeutic dose is clinically tolerated. Rapid desensitization (RD) protocols have been published for IgE- and non-IgE-mediated mast cell reactions caused by chemotherapeutic and biologic agents such as sulfonamides, aspirin and non-β-lactam antibiotics, but the mechanism of tolerance is still largely unknown (1,4,5).
A recently classified syndrome, mast cell activation syndrome (MCAS), is characterized by episodes of multiple symptom constellations that can be explained by mast cell mediator release. Symptoms usually affect more than one organ system and consist of cutaneous and mucus membrane involvement such as urticaria, angioedema conjunctival injection, pruritus or flushing; gastrointestinal involvement with nausea, vomiting, diarrhea, abdominal cramping; cardiovascular involvement with hypotensive syncope or near syncope, tachycardia, and respiratory system involvement ranging from nasal stuffiness to stridor to wheezing. Documentation of response to treatment with medications aimed at mast-cell mediators, such as H1 and H2 histamine receptor antagonists, anti-leukotrienes, or mast cell stabilizers is also used to make the diagnosis of MCAS. Mast cell disorders including systemic mastocytosis (SM) and monoclonal mast cell disorder (MCAD) may be similar in presentation and should be excluded before diagnosis of MCAS is assigned. Laboratory data to support the diagnosis of MCAS include increased serum tryptase levels persistently >15 ng/mL at baseline, increased serum tryptase levels during symptomatic periods > baseline values, and elevated levels of 24 h our urinary histamine metabolites, prostaglandin D2 (PGD2) or its subunit 11-Beta-prostaglandin F2 alpha (6,7).
Known triggers differ in each patient, but MCAS can be exacerbated by physical factors such as heat, cold, pressure or friction, hymenoptera stings, fever or infection, exercise, invasive procedures (e.g., general anesthesia, biopsy, endoscopy), exposure to alcohol, drugs such as antibiotics, NSAIDs, narcotics or neuromuscular blocking agents, radiocontrast media and/or emotions/stress (8-10). Avoidance of these factors, though at times impossible, are key to limiting episodes. Current MCAS management consists of histamine receptor antagonists and mast cell stabilizers. Epinephrine intramuscular (IM) autoinjector should be prescribed to all patients and patient should be instructed on instrumentation and administration when symptoms present (11,12). New research is determining the success of biologics in controlling or mitigating episodic symptomatology (9,12). In patients with consistent symptoms across multiple organ systems, MCAS may be the underlying unifying pathology that may be overlooked when organ-specific diagnoses are sought.
There are many overlapping criteria between anaphylaxis and the MCAS episodes. As in the general population, patients with MCAS may also have true allergies to medications. Furthermore, patients will require new medications based on clinical circumstances, which raises a question—how to safely administer medications in patient at risk of MCAS resulting in potential anaphylaxis-like reaction? We present a case that illustrates the difficulties in such decisions and describes a possible approach.
Case presentation
A 17-year-old male with past medical history of MCAS (diagnosed 2 years prior), dysautonomia, Ehlers Danlos Type III and multiple drug allergies was admitted to the Pediatric Intensive Care Unit after presentation with shortness of breath and a cough with radiographic evidence of pneumonia. Patient endorsed a history of reactions to ceftriaxone and azithromycin consistent with anaphylaxis. Furthermore, the patient reported a history of reactions to multiple medications on first exposure. A desensitization protocol with ceftriaxone was chosen in collaboration with PICU staff, infectious disease, and immunology in the setting of a documented beta lactam allergy (13). The patient successfully completed the protocol as noted in Table 1, but did not have clinical improvement, which prompted a PCR nasal swab positive for Chlamydia pneumoniae. Based on continued hypoxia and lack of symptom resolution, the decision was made to desensitize to azithromycin in the face of a documented past anaphylaxis. The patient tolerated his first three full doses of azithromycin without any hemodynamic instability or signs of anaphylaxis (Table 2). Treatment with azithromycin resulted in improvement of dyspnea, cough and crackles on auscultation. After his fourth day of antibiotic treatment, he was found have pruritus in his left arm and bilateral shoulders, as well as small papules and erythema on his right upper back without confluence on his left arm at the infusion site an hour after his dose. He was given one dose intravenous diphenhydramine and two doses of solumedrol. The pruritus and erythematous rash resolved and decision was made to not give his fifth day of azithromycin course given the encouraging degree of clinical improvement of pneumonia.
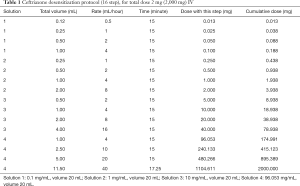
Full table
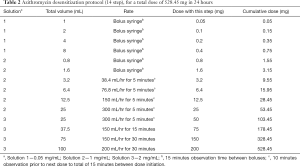
Full table
Discussion
As discussed, a number of questions arise when faced with a patient with MCAS who may require administration of a potentially dangerous medication. Do we desensitize patients due to the fact that anaphylactic or anaphylactoid reaction may occur in someone with a history of multiple previous reactions on first exposure? Does desensitization last in these patients? Is there laboratory data we can measure to distinguish if the patient is undergoing anaphylactoid or true anaphylaxis?
In our patient with pneumonia and a history of reactions to ceftriaxone and azithromycin, protocols consisting of progressive doses of these medications every 15 to 30 minutes until a full therapeutic dose, were clinically tolerated but resulted in a mild reaction on subsequent dosing of azithromycin. Both of the medications had documented anaphylaxis-like reactions in the past. Overall, patients with MCAS produce a lifelong difficulty in disease management. Our case argues that desensitization may be a safe way of administering first dose, but subsequent doses probably will need to be done under observation, given the possibility of further reactions, highlighted by the breakthrough reaction on the last dose of azithromycin in our patient.
The prospective and retrospective management of MCAS can be similar to that of the patient with multiple drug allergies which is stratified by risk factors (14). A good place for the practitioner to start is with the details of past reactions. Timing, distribution, past exposures and route of administration help to determine severity and type of reaction. One should try to differentiate if the reactions are immune related and determining if reaction is predictable versus unpredictable (15). Predictable reactions include overdoses, side effects, secondary effects, drug–drug interactions which can be altered by the chosen medications. Unpredictable reactions may be non-immune which include drug intolerances, drug idiosyncrasies and pseudoallergic reactions. When immune mediated, the reaction will typically fit into the Type I–IV classification (14,16,17).
In patients with MCAS, the mechanism of sensitivity to a particular drug may not be IgE-mediated. We do not know if our patient had IgE to ceftriaxone or azithromycin, but we speculate that his reactions were due to activation of mast cells given previous clinical presentation, but our case argues that desensitization still may be considered, with some precautions in light of a possibility of reaction on subsequent dosing. Classically, the mechanism of desensitization depends on antigen-specific mast cell and basophil desensitization, which is poorly understood, but very low dose exposure appears to make these cells tolerant to the specific antigen. To stimulate mast cells to degranulate in IgE-mediated fashion, IgEs need to be cross-linked on the surface of mast cell—in a very low-dose exposure, the amount of antigen may not be enough for cross-linking, and may send inhibitory signal, rather than stimulating signal (18). In MCAS, since we assume that the sensitivity is non-specific (non-IgE), the question of how desensitization works can be raised and discussed with more research (19). However, when necessary, desensitization may potentially be employed despite the fact that the exact factors are poorly understood and the procedure does incur substantial cost, monitoring, and time.
Skin drug allergy testing in the setting of MCAS may result in false-positive reading since skin manipulation can trigger mast cell degranulation; alternatively, negative result may not rule out possibility of MCAS flare when the medication is administered systemically. Thus, if the patient’s history is not concerning for systemic reaction, a cautious graded challenge to the medication in question may be considered if there are no other contraindications such as history of Steven-Johnson Syndrome, toxic epidermal necrolysis or other reactions that would preclude re-exposure to the medication. We would advise against a drug provocation test or incremental challenge in a MCAS patient with documented prior anaphylaxis (15). These can be performed when there is a low likelihood of an IgE-mediated hypersensitivity (15,20). Presence of MCAS would not preclude starting novel medications, however, we would recommend that the first dose be given in an office setting with access to epinephrine and trained personnel rather than isolated at home, especially if the medication is a known mast-cell activator.
Conclusions
Management of drug reactions in patients with MCAS is complicated and not well researched. Our case illustrates that desensitization for documented immediate-type reactions may be employed, however caution and possibly inpatient monitoring of subsequent doses should be considered.
Acknowledgements
We thank Dr. Laura Bauler and Dr. Dilip Patel for review and edits of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
References
- American Academy of Allergy, Asthma and Immunology, the American College of Allergy, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105:259-73. [Crossref] [PubMed]
- Lieberman P, Garvey LH. Mast Cells and Anaphylaxis. Curr Allergy Asthma Rep 2016;16:20. [Crossref] [PubMed]
- Pichler WJ, Hausmann O. Classification of Drug Hypersensitivity into Allergic, p-i, and Pseudo-Allergic Forms. Int Arch Allergy Immunol 2016;171:166-79. [Crossref] [PubMed]
- Castells Guitart MC. Rapid drug desensitization for hypersensitivity reactions to chemotherapy and monoclonal antibodies in the 21st century. J Investig Allergol Clin Immunol 2014;24:72-9; quiz 2 p following 79.
- Gupta M, Gomes JM, Irizarry J, et al. The use of standardized drug desensitization protocols at a pediatric institution. J Allergy Clin Immunol Pract 2017;5:834-6.e5. [Crossref] [PubMed]
- Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: Proposed diagnostic criteria. J Allergy Clin Immunol 2010;126:1099-104.e4. [Crossref] [PubMed]
- Frieri M. Mast Cell Activation Syndrome. Clin Rev Allergy Immunol 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Picard M, Giavina-Bianchi P, Mezzano V, et al. Expanding spectrum of mast cell activation disorders: monoclonal and idiopathic mast cell activation syndromes. Clin Ther 2013;35:548-62. [Crossref] [PubMed]
- Bonamichi-Santos R, Castells M. Mast Cell Activation Syndromes. Curr Treat Options Allergy 2016;3:384-400. [Crossref]
- Hamilton MJ, Hornick JL, Akin C, et al. Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations. J Allergy Clin Immunol 2011;128:147-52.e2. [Crossref] [PubMed]
- Frieri M, Patel R, Celestin J. Mast cell activation syndrome: a review. Curr Allergy Asthma Rep 2013;13:27-32. [Crossref] [PubMed]
- Molderings GJ, Brettner S, Homann J, et al. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol 2011;4:10. [Crossref] [PubMed]
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, et al. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009;8:418-24. [Crossref] [PubMed]
- Khan DA. Treating patients with multiple drug allergies. Ann Allergy Asthma Immunol 2013;110:2-6. [Crossref] [PubMed]
- Schnyder B. Approach to the Patient with Drug Allergy. Immunol Allergy Clin North Am 2009;29:405-18. [Crossref] [PubMed]
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol 2011;127:S67-73. [Crossref] [PubMed]
- Farnam K, Chang C, Teuber S, et al. Nonallergic drug hypersensitivity reactions. Int Arch Allergy Immunol 2012;159:327-45. [Crossref] [PubMed]
- Torres MJ, Mayorga C, Blanca-López N, et al. Hypersensitivity reactions to beta-lactams. EXS 2014;104:165-84. [Crossref] [PubMed]
- Castells MC. A New Era for Drug Desensitizations. J Allergy Clin Immunol Pract 2015;3:639-40. [Crossref] [PubMed]
- Rerkpattanapipat T, Chiriac AM, Demoly P. Drug provocation tests in hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol 2011;11:299-304. [Crossref] [PubMed]
Cite this article as: Staso P, Leonov A. Drug desensitization in 17-year-old male with Mast Cell Activation Syndrome, pneumonia, and antibiotic hypersensitivities. AME Case Reports 2017;1:7.


