Malignant melanoma of parotid gland in a child—our unique experience
Introduction
Primary parotid gland malignant melanoma (PGMM) is rare, accounting for less than 0.7% of all parotid gland malignancies (1). Childhood PGMM is extremely uncommon and very few cases have been reported in the literature. Management of PGMM is often complicated owing to difficulty in establishing diagnosis and the inherent poor prognosis of this condition.
Case presentation
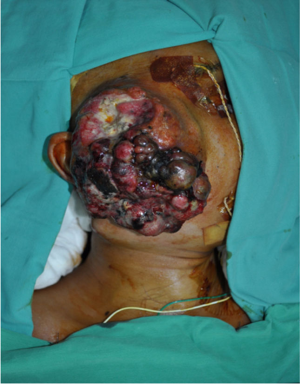
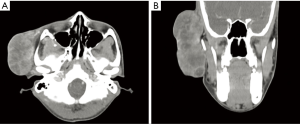
A boy aged 13 presented with right parotid gland swelling for 6 months. It initially appeared as a small skin-coloured pimple which had rapidly increased in size. A large swelling measuring 15 cm × 10 cm (Figure 1) featuring irregular surface, hard consistency and immobility was evident. There were areas of ulceration and necrosis. The lesion was tender and bled with gentle touch. Right-sided level III cervical lymphadenopathy was demonstrable with the largest node being hard, non-tender, mobile and measuring 3 cm × 4 cm. Ipsilateral Facial nerve function was intact. There was no trismus and oral examination revealed medially displaced right lateral pharyngeal walls. Rest of the examination including assessment of ears, nose and laryngoscopy was insignificant. A full external examination revealed no other lesions especially skin lesions. CT scan demonstrated a heterogeneously enhancing, lobulated, superficial parotid mass lateral to right zygomatic arch measuring 9.3×4.9×6.3 cm3 with multiple hypo-dense necrotic foci. It was poorly demarcated (Figure 2). The scan also identified a 3.0×3.2×4.2 cm3 mass with multiple necrotic foci at level III cervical lymph node region. There was no evidence of other distant metastatic lesions.
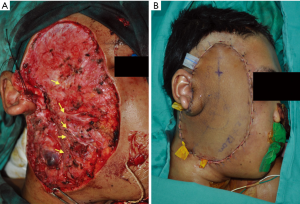
Following a multidisciplinary meeting decision a wide local excision was performed. A fungating mass arising from superficial parotid gland with skin ulceration, necrosis and haemorrhage was excised alongside with skin and fascia. Excised tumour tissue measured 12×7×9 cm3. Facial nerve and all five branches were identified, unaffected and preserved (Figure 3). The deep lobe of parotid gland appeared uninvolved. Concurrent selective cervical lymph node dissection (CLND) (levels II and III) revealed a lobulated, necrotic level III mass which was excised. The wound was reconstructed with left anterolateral thigh flap. Post-operative recovery was uncomplicated and patient was discharged after 6 days.
Histopathological examination showed multi-nodular, uncircumscribed infiltrative malignant tumour with highly pleomorphic cells. Intranuclear inclusions, binucleation and high number of mitoses were seen. The tumour cells showed diffuse positivity towards Melan-A, Vimentin and S100 but stained negative for HMB45. Superior, inferior, posterior and deep margins were infiltrated by tumour cells. The overlying skin showed extensive destruction and loss of architecture secondary to tumour cells invasion.
Post-operative radiotherapy was planned however new loco-regional disease recurrence during waiting period necessitated combination chemotherapy of dacarbazine and cisplatin instead. Unfortunately, our patient succumbed to disease at 7 months post-surgery.
Discussion
Incidence of MM in paediatric population is 1–2% and 3–4% of all childhood malignancies are MM. Boys usually present at a more advanced stage which is associated with poorer prognosis (2). MM account for 0.68% of all parotid malignancies (1). Commonest cause of PGMM is metastasis from other sites especially from head and neck cutaneous primaries (1,3). These lesions spread to preauricular-parotid regional lymph nodes that drain a large area of head and neck and subsequently involve the adjacent parotid gland. Direct extension of an adjacent cutaneous MM to parotid gland is another mode of metastasis which has been described in literature. Prayson and Sebek found in their review of twelve PGMM cases that two patients had simultaneous cutaneous and parotid gland lesions (4). Infrequently, PGMM with an unknown primary could be a result of regression of the initial lesion (5).
Primary PGMM is not an impossibility and a few theories regarding this have been discussed in literature. Takeda in 1997 reported that melanocytes within interlobular duct of parotid gland could result in PGMM (6). Greene and Bernier theorised that PGMM could arise from melanoblasts that may be present in parotid gland as it is embryologically formed from invagination of melanoblasts-containing buccal epithelium (7). Nevertheless, primary PGMM is a diagnosis of exclusion and these four criteria should be fulfilled for its diagnosis (8):
- The bulk of tumour contained within parotid gland;
- The tumour contains no identifiable lymph node tissue;
- No evidence of other MM lesions in the body;
- No previous excision of MM or suspicious pigmented lesion.
Gao et al. in 2008 reported a primary PGMM case and the diagnosis was based on criteria above (8). These criteria were met in our patient for a diagnosis of primary PGMM.
The gold diagnostic criterion for MM is presence of intracellular melanin pigmentation which is found in only 40–60% of cases (3). MM cells, via immunohistochemistry staining, would exhibit presence of S100 and HMB45 proteins in most cases (9). In our case positivity for S100 and Melan-A confirmed the diagnosis.
Total parotidectomy is considered the best treatment option for PGMM, however necessity of this invasive intervention is debatable as the tumour carries poor prognosis and the intervention is unlikely to prolong life. Wang et al. in 1999 recommended superficial parotidectomy with CLND for PGMM as there is no added survival advantage of a radical parotidectomy (10). A superficial parotidectomy was performed in our patient; this was deemed sufficient intraoperatively as the tumour was well demarcated and deep parotid lobe appeared uninvolved. This decision was also supported by radiological evidence of tumour confinement to superficial lobe. CLND alongside parotidectomy is imperative as occult MM metastasis rate to these nodes is high, ranging from 21–71% (11). Regardless of clinical and radiological findings, tumour staging is more accurate with a combined parotidectomy and CLND.
Adjuvant postoperative radiotherapy to both parotid and neck region confers approximately 6% reduction of recurrence risk. Radiation also reduces burden of microscopic disease (11).
Patients with PGMM have a poor prognosis despite best treatment efforts. Metastasis to cervical lymph nodes results in poorer prognosis. High incidence of distant tumour spread in PGMM contributes to significant reduction in survival rate (3). In addition, involvement of skin at presentation is another important predictive factor that leans towards poor outcome (12). This was evident in our patient.
Acknowledgements
The authors would like to thank Nur Asyilla Che Jalil (Department of Pathology, University of Science Malaysia, Kelantan, Malaysia); Sharifah Emilia Tuan Sharif (Department of Pathology, University of Science Malaysia, Kelantan, Malaysia); and Shahrizal Mustaffa (Department of Oncology, University of Science Malaysia, Kelantan, Malaysia).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the parent of patient for publication of this case report and any accompanying images.
References
- Bahar M, Anavi Y, Abraham A, et al. Primary malignant melanoma in the parotid gland. Oral Surg Oral Med Oral Pathol 1990;70:627-30. [Crossref] [PubMed]
- Lewis KG. Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg 2008;34:152-9. [Crossref] [PubMed]
- Bron LP, Traynor SJ, McNeil EB, et al. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope 2003;113:1070-5. [Crossref] [PubMed]
- Prayson RA, Sebek BA. Parotid gland malignant melanomas. Arch Pathol Lab Med 2000;124:1780-4. [PubMed]
- Santini H, Byers RM, Wolf PF. Melanoma metastatic to cervical and parotid nodes from an unknown primary site. Am J Surg 1985;150:510-2. [Crossref] [PubMed]
- Takeda Y. Melanocytes in the human parotid gland. Pathol Int 1997;47:581-3. [Crossref] [PubMed]
- Greene GW Jr, Bernier JL. Primary malignant melanomas of the parotid gland. Oral Surg Oral Med Oral Pathol 1961;14:108-16. [Crossref] [PubMed]
- Gao N, Li LJ, Li Y, et al. Primary amelanotic malignant melanoma of the parotid gland: a case report. J Int Med Res 2008;36:1435-9. [Crossref] [PubMed]
- Saqi A, McGrath CM, Skovronsky D, et al. Cytomorphologic features of fine-needle aspiration of metastatic and recurrent melanoma. Diagn Cytopathol 2002;27:286-90. [Crossref] [PubMed]
- Wang BY, Lawson W, Robinson RA, et al. Malignant melanomas of the parotid: comparison of survival for patients with metastases from known vs unknown primary tumor sites. Arch Otolaryngol Head Neck Surg 1999;125:635-9. [Crossref] [PubMed]
- Ballo MT, Garden AS, Myers JN, et al. Melanoma metastatic to cervical lymph nodes: Can radiotherapy replace formal dissection after local excision of nodal disease? Head Neck 2005;27:718-21. [Crossref] [PubMed]
- Gross M, Maly B, Maly A, et al. Metastatic malignant melanoma involving the parotid lymph node region: a clinicopathologic report of 5 cases. J Oral Maxillofac Surg 2008;66:809-13. [Crossref] [PubMed]
Cite this article as: Apparau D, Apparau H, Mohamad I, Bhavaraju VM. Malignant melanoma of parotid gland in a child—our unique experience. AME Case Rep 2018;2:4.