Granulomatosis with polyangiitis causing subglottic stenosis—two cases and their management
Introduction
Granulomatosis with polyangiitis (GPA) is characterised by vasculitis of small and medium sized blood vessels and granulomatous lesions of the respiratory tract (1). The aetiology is unclear, however it is thought to be due to an autoimmune process with about 92% of patients with the disease being antineutrophilic cytoplasmic antibodies (ANCA) positive (2). There is also growing evidence of environmental factors, genetic predisposition and microbial colonisation playing a role (1,3).
It can affect any of the major systems giving rise to ear, nose, throat, respiratory, cardiac ophthalmic, musculoskeletal, renal, neurological, and dermatological symptoms and signs. Patients normally present in early adulthood, more commonly in the winter months (4). Seventy percent of patients with GPA present with ear, nose or throat symptoms. These include nasal congestion, crusting, epistaxis, nasal septal perforation and nasal saddle deformity (5). Lesions in the airway can lead to subglottic stenosis with resultant airway obstruction (6).
Treatment of the disease complicated by subglottic stenosis is not straightforward and the benefits and risks of options including medical and surgical management need to be weighed up and tailored to each individual case. We describe two cases of GPA complicated by airway obstruction due to subglottic stenosis and their management.
Case presentation
Case 1
A 23-year-old female with a 4-year history of previously diagnosed sarcoidosis presented acutely (Gravida 1 Para 0) at 38 weeks with increasing stridor and difficulty breathing. Assessment revealed that she had stridor at rest, was unable to lie flat and her exercise tolerance was significantly reduced. She had been treated with long-term oral prednisolone, but unfortunately she was non-compliant during her pregnancy. Evaluation of the airway revealed significant subglottic stenosis and a multi-disciplinary team was called to plan her further management. The patient refused an elective tracheostomy and wanted to consider a trial of labour. A trial of labour was considered too risky by the Obstetric, ENT & Anaesthetic teams without a plan to manage the airway and the risks of an emergency tracheostomy were explained to the patient.
Ultimately it was agreed that the patient would have a planned lower segment caesarean section under general anaesthesia and an elective tracheostomy. It was felt safer to deliver the baby first prior to the tracheostomy as it was going to be a difficult tracheostomy and would be easier without the increased oxygen demand of the foetus & left lateral tilt position.
Lumbar epidural was cited in sitting position for anaesthesia in theatre during which the patient’s oxygen saturations dropped to 86%. Anaesthesia was induced with inhalation induction, Grade 1 view laryngoscopy. However, we were unable to pass a microlaryngoscopy tube (MLT) size 5.0 & could only just pass an Aintree Intubation Catheter (19 Fr, ID 4.7 mm) down through the tracheal stricture.
The epidural was topped up to provide analgesia. Anaesthesia was maintained with inhalation agent supplemented with intravenous agent. The caesarean section was performed with a live baby delivered. The patient desaturated towards the end of caesarean section, so ventilation was assisted. A surgical tracheostomy was performed which was difficult due to engorged thyroid & bleeding within the trachea from the lesion. During this process the Aintree catheter became blocked & she suffered a PEA arrest due to hypoxia, eventually a MLT was passed into the trachea & ventilation was successful with oxygenation saturations improved to 90%. Using the guidewire from a Melker cricothyroidotomy kit, the MLT was successfully replaced by size 6 tracheostomy tube.
She was stabilized and transferred to the intensive care unit, where sedation and ventilation were continued with the addition of therapeutic hypothermia as per NICE guidelines for neuroprotection for 24 hours post cardiac arrest (7). The patient was found to have no neurological deficit on weaning off sedation at 36 hours post cardiac arrest.
The post-operative high resolution computed tomography scan (HRCT) (Figure 1) showed diffuse circumferential oedema and thickening of the mucosa and submucosal tissues of the airway. There was almost complete occlusion both at and immediately above the glottis level over a 2-cm craniocaudal length. The subglottic area showed only mild circumferential narrowing of the airway. It was felt this was likely an exacerbation of sarcoidosis, though evaluation of the literature suggested sarcoid improves in pregnancy, whereas GPA worsens, and the patient was recommenced on steroid treatment.

Following a step down from ICU, she underwent micro laryngoscopy evaluation of the airway and biopsy of the stenosed tissue. Biopsy confirmed GPA. She was referred to the rheumatologists who after considerable deliberation and counselling commenced treatment on rituximab infusions and oral steroids.
The patient clinically improved on steroids and was discharged with a tracheostomy. She was referred to a tertiary ENT centre for further evaluation of the airway and subsequent balloon dilatation.
The following year, she underwent successful decannulation. This was complicated by a persistent trachea-cutaneous fistula, which was surgically closed. GPA was controlled mostly following this by repeated rituximab infusions during exacerbations and long term prednisolone.
However two years after her initial diagnosis, she developed saddling of the nose, nasal crusting and worsening shortness of breath and bilateral vocal cord palsy, with the right vocal cord fixed in the paramedian position. She was also found to have signs of subglottic and tracheal stenosis on flexible nasoendoscopy. A trial of rituximab infusions failed to improve her symptoms, and thus required a further direct laryngo-tracheo-bronchoscopy and dilatation of the subglottic and tracheal stenosis. Following this, the patient’s management was modified to azathioprine and prednisolone. To date, this appears to have her condition under control, with no further shortness of breath nor stridor and vocal cord movement has returned to normal.
Case 2
Our second case is a 40-year-old male with a ten year history of quiescent, well controlled GPA, who presented to hospital with worsening shortness of breath, stridor and cough. Fibre-optic nasoendoscopy showed diffuse oedema suggestive of silent reflux and a subglottic narrowing at the 2nd tracheal ring. The patient’s steroid dose was increased and he was started on omeprazole as it was felt this was an exacerbation of his GPA with associated reflux. He seemed to improve.
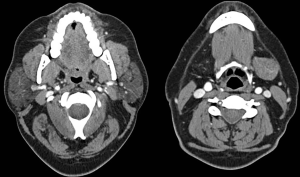
Two months later however, his symptoms returned. He was admitted because of increasing stridor and his symptoms improved with intravenous steroids and two rituximab infusions. Despite this, he returned a month later with worsening of his shortness of breath. Flexible nasoendoscopy demonstrated significant subglottic stenosis with some mild interarytenoid oedema with an adequate airway. CT (Figure 2) showed that there was no further tracheal stenosis demonstrated. It was decided at that point to admit the patient for urgent cyclophosphamide infusion and intravenous steroids. He improved with this treatment and was discharged with an elective second cyclophosphamide infusion a month later.
After a total of four cyclophosphamide infusions over 6 months, a repeat CT scan showed no evidence of subglottic inflammation and nasoendoscopy demonstrated no evidence of granulation tissue. For over a year repeated cyclophosphamide infusions stabilized his GPA with no further stridor or breathlessness.
He developed breathlessness again gradually despite this. Examination showed this to be due to recurrence of subglottic stenosis. He later underwent an elective balloon dilatation at a tertiary centre of his subglottic stenosis with transtracheal triamcinolone injection whilst his condition was stable. To date, his condition appears stabilized with oral steroids and regular cyclophosphamide infusions.
Discussion
Over 90% of patients with GPA present with upper and/or lower respiratory tract symptoms and about 20% develop subglottic stenosis (5). Granulomatous lesions giving rise to airway obstruction of the larynx and trachea can lead to gradual or acute airway obstruction and could result in death (6,7). Stenosis can occur at sites ranging from the larynx to the bronchi, the most common being subglottic stenosis (8). Airway stenosis is more common in women (8).
Airway obstruction from GPA can be subdivided into acute and chronic phases (9). The acute phase is due to fixation of the vocal folds from inflammation of the laryngeal joints. Symptoms of the acute phase tend to improve with medical therapy (9).
The acute presentation can be of a patient presenting for the first time, a relapse in a patient with a known diagnosis of GPA or a patient with symptoms exacerbated by acute infection (4). As its symptoms are similar to other common otolaryngological or respiratory diseases, a high index of suspicion is required for a timely and accurate diagnosis.
Blood tests may reveal a normocytic anaemia with raised inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). ANCA would be positive in serological essays (1). Plain radiographs are limited in detecting airway obstruction and thus radiological imaging should be in the form of computed tomography or magnetic resonance imaging (1,9). Flexible nasoendoscopy would be able to detect upper airway lesions and subglottic stenosis but may miss more distal stenosis.
Although biopsies often are of non-specific low-diagnostic accuracy, often just showing chronic inflammation, a positive biopsy of granuloma or vasculitis is strongly supportive of the diagnosis and thus recommended when possible (10,11). Biopsy samples are most useful diagnostically if taken from areas of active disease, lung or renal lesions (1). It may be unnecessary if diagnosis is made confident by other tests and clinical examination and biopsies are difficult to attain (1,11).
Treatment is predominantly medical in the form of corticosteroids and cytotoxic agents. Aggressiveness and type of pharmacological therapy depends on the severity grading of the disease which is dependent on whether the disease is non-organ threatening; organ or life threatening; rapidly progressive renal failure or pulmonary haemorrhage; or refractory, in remission or a relapse (12). Medical treatment of organ or life threatening disease, as was the case in both patients, is treated with a combination of corticosteroid and rituximab or cyclophosphamide. Remission maintenance therapy involves corticosteroid and one of rituximab, azathioprine, methotrexate or mycophenolate mofetil (10).
The late chronic phase of airway obstruction is usually caused by subglottic stenosis (9). Although thought to have developed gradually over time (9), patients may present with acute airway obstruction rather than a gradual decline. Subglottic stenosis is in the form of an anterior web or circumferential ring (4) with a pale smooth surface (9). Biopsies of the stenosis may show granulation tissue consistent with GPA or just scar tissue (9). Acute presentations may benefit from pharmacotherapy, however many would require surgical treatment (4).
There are various surgical options, the most common being endoscopic balloon dilatation. This procedure has been found to be safe (8). Dilatation can also be performed using a bougie although it may have a higher rate of postoperative haemorrhage (8). Concurrent use of high dose corticosteroids and increased time from stenosis to dilatation appears to result in a higher rate of event-free survival compared to dilatation alone (13).
Other surgical options include endoscopic laser carbon dioxide or Nd-YAG resection (14), argon plasma coagulation, and cryotherapy. Intralesional Mitomycin-C, corticosteroid, or Alemtuzumab has also been described and can be done at the same time as endoscopic dilatation. There is a risk of granulation and scarring after local Mitomycin-C injection, which may require subsequent resection (8). Stents tend not to be used as they contribute to ongoing local inflammation (8). Restenosis over the stents may require their subsequent removal (8).
Unfortunately, surgical treatment may only have temporary benefits as patients tend to relapse in terms of subglottic or tracheal stenosis despite medical therapy post-surgery (8,15,16). These relapses affecting the airway appear to evolve independent of other organ involvement (13,15). Thus most of these patients require multiple procedures before long-term stable airway patency is achieved (8,16).
Tracheostomies are reserved for emergency cases or where there is severe destruction of the laryngeal or tracheal anatomy (9). Excision of stenosis with end-to-end repair is another described option that is rarely used.
Conclusions
GPA can cause airway obstruction in the form of subglottic stenosis in a significant proportion of patients. Treatment involves medical and surgical therapy. Surgical therapy usually has to be repeated due to restenosis of the airway despite ongoing medical treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Tracy CL, Papadopoulos PJ. Granulomatosis with Polyangiitis (Wegener Granulomatosis). Available online: (accessed 8 March 2018).http://emedicine.medscape.com/article/332622-overview
- Finkielman JD, Lee AS, Hummel AM, et al. ANCA are detectable in nearly all patients with active severe Wegener's granulomatosis. Am J Med 2007;120:643.e9-14. [Crossref] [PubMed]
- Stegeman CA, Tervaert JW, Sluiter WJ, et al. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med 1994;120:12-7. [Crossref] [PubMed]
- Martinez Del Pero M, Sivasothy P. Vasculitis of the upper and lower airway. Best Pract Res Clin Rheumatol 2009;23:403-17. [Crossref] [PubMed]
- Langford CA, Hoffman GS. Rare diseases.3: Wegener's granulomatosis. Thorax 1999;54:629-37. [Crossref] [PubMed]
- Martinez Del Pero M, Rasmussen N, Chaudhry A, et al. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener's). Eur Arch Otorhinolaryngol 2013;270:345-54. [Crossref] [PubMed]
- Matt BH. Wegener's granulomatosis, acute laryngotracheal airway obstruction and death in a 17-year-old female: case report and review of the literature. Int J Pediatr Otorhinolaryngol 1996;37:163-72. [Crossref] [PubMed]
- Martinez Del Pero M, Jayne D, Chaudhry A, et al. Long-term outcome of airway stenosis in granulomatosis with polyangiitis (Wegener granulomatosis): an observational study. JAMA Otolaryngol Head Neck Surg 2014;140:1038-44. [Crossref] [PubMed]
- Rasmussen N. L24. Local treatments of subglottic and tracheal stenoses in granulomatosis with polyangiitis (Wegener's). Presse Med 2013;42:571-4. [Crossref] [PubMed]
- Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016;75:1583-94. [Crossref] [PubMed]
- Devaney KO, Travis WD, Hoffman G, et al. Interpretation of head and neck biopsies in Wegener's granulomatosis. A pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990;14:555-64. [Crossref] [PubMed]
- Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009;68:310-7. [Crossref] [PubMed]
- Terrier B, Dechartres A, Girard C, et al. Granulomatosis with polyangiitis: endoscopic management of tracheobronchial stenosis: results from a multicentre experience. Rheumatology (Oxford) 2015;54:1852-7. [Crossref] [PubMed]
- Gouveris H, Karaiskaki N, Koutsimpelas D, et al. Treatment for adult idiopathic and Wegener-associated subglottic stenosis. Eur Arch Otorhinolaryngol 2013;270:989-93. [Crossref] [PubMed]
- Girard C, Charles P, Terrier B, et al. Tracheobronchial Stenoses in Granulomatosis With Polyangiitis (Wegener's): A Report on 26 Cases. Medicine (Baltimore) 2015;94:e1088. [Crossref] [PubMed]
- Tanna N, Boone J. Otolaryngologic Manifestations of Wegener Granulomatosis. Available online: (accessed 8 March 2018).https://emedicine.medscape.com/article/858001-overview
Cite this article as: Blackabey V, Gan RW, Buglass H, Kaul V, Ward VM. Granulomatosis with polyangiitis causing subglottic stenosis—two cases and their management. AME Case Rep 2018;2:17.