A rare case of intralobar pulmonary sequestration: combined endovascular and video-assisted thoracoscopic approach
Introduction
Pulmonary sequestration is a congenital malformation characterized by cystic, non-functioning embryonic lung tissue with vascularization of an abnormal systemic artery (1). It’s rare and varies widely in clinical presentation and severity, depending mostly on the degree of lung involvement and the location in the thoracic cavity (2). Blood supply originates generally from the systemic circulation via the descending thoracic aorta branches and, rarely, via an aberrant branch from the abdominal aorta (2). In the last few years, the development of angiographic techniques allowed its application to cases of pulmonary sequestrations. We present a case of intralobar pulmonary sequestration located in the lower lobe of the right lung treated with video-assisted thoracoscopic surgery (VATS) after endovascular embolization.
Case presentation
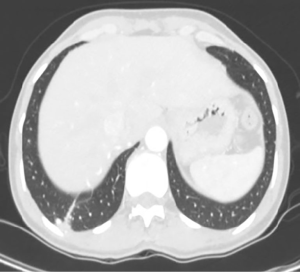
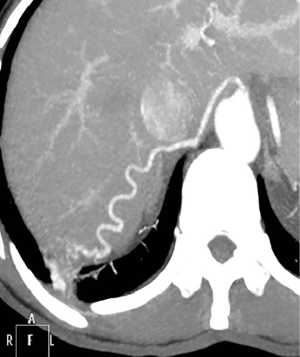
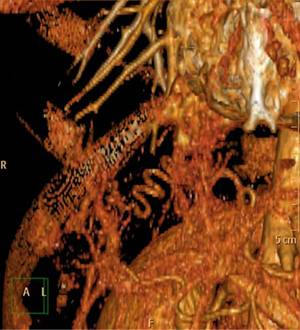
A 47-year-old female patient presented to our Institution for persistent chest wall pain. Her past medical history included Raynaud’s disease. Physical examination, cardiological and laboratory test findings were otherwise negative. Chest computed tomography (CT) revealed at the level of the right lower pulmonary lobe of a ground glass area, with a diameter about of 4 cm × 3 cm supplied by an aberrant arterial branch of the proximal tract of the abdominal aorta (Figure 1) crossing the diaphragm to perfuse the right lower lobe (Figure 2) as evidenced also by the 3D reconstruction of the CT (Figure 3). After multidisciplinary discussion the patient was scheduled for arteriography with selective embolization of the aberrant arterial branch followed by thoracoscopic resection of the affected part of the lower right lobe. This approach was chosen in order to reduce a potentially lethal intra-abdominal hemorrhage.
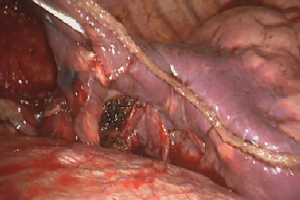
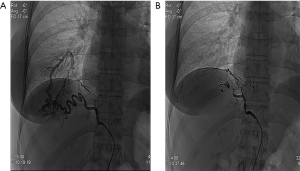
Transcatheter arterial angiography revealed the aberrant branch originating from the celiac trunk and crossing the diaphragm and embolization was performed using gelatin sponge particles and two metallic coils (Figure 4). The day after, a VATS approach using three ports was performed. The posterior basal segment was partly hepatized and the aberrant branch was identified and divided after positioning titanium clips. Subsequently, a wedge resection of the affected lung was performed (Figure 5) and a single chest tube was placed at the end of the procedure. We decided to perform wedge resection instead of anatomical resection because of the small size of the lesion. The patient was discharged on the postoperative day 4, with no complications. The pathology examination confirmed the presence of an intralobar pulmonary sequestration. The patient remains in good health 12 months after surgery.
Discussion
Pulmonary sequestration comprises 0.15% to 6% of all congenital pulmonary malformations (3) and is characterized by normal, non-functioning lung tissue without connection with the bronchial tree receiving its blood supply from the systemic circulation (2). Some abnormalities and concomitant malformations have been reported in the presence of pulmonary sequestration (diaphragmatic hernia 36%, herniae with diaphragmatic defects 19%, relaxation of the diaphragm 5%, lung anomalies 13%, oesophago-tracheal fistula 2%, epiphrenic diverticulum 3%, vitium cordis 6%, pericardial anomalies 4%, funnel chest 1%, oesophageal duplication 1%, megacolon 1%, other anomalies 11%) (3). It is more commonly observed in the left lung, mainly in the lower lobes (1), usually affecting the posterior basal segment. The aberrant artery in intralobar sequestration originates in 74% of all cases from the thoracic aorta, and in 14% there was more than one anomalous artery (3). A single artery supplies the majority of cases, and 21% of cases show two or more arteries (4). Chest CT is the diagnostic technique of choice (1) and it can show mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and pneumonic lesions (8%) (4). Some radiographic findings in intralobar lung sequestration are: cystic area, fluid level, homogeneous shadow, inhomogeneous shadow, retrocardiac localization and multiple fluid levels (3). In most cases angiography is not necessary since the feeding arteries of a sequestration can be easily identified using conventional CT images (5).
Surgical resection is the gold standard of therapy (6) and anatomical resection is the procedure of choice yielding excellent long-term results (2).
In most cases the inflammatory changes in the area of the sequestration due to recurrent infections hide the aberrant artery in the scar tissue. Therefore, the identification of the aberrant artery or arteries should be done carefully at the beginning of the procedure (5). New technologies developed in the past few decades, e.g., preoperative interventional angiology procedures and video-assisted lung resection have changed the management of the disease (6). However, the majority of lobectomies are still performed via thoracotomy and there are only few reports on VATS approach for pulmonary sequestration reporting its feasibility despite the possible technical difficulties due to inflammatory changes (1,5).
Embolization has been reported exceptionally for the treatment of severe haemoptysis in Pryce type I sequestration while surgery remains the gold standard for treatment for Pryce types II and III (7,8) (Table 1). Embolization can be performed using polyvinyl alcohol (PVA) particles, gelfoam and/or platinum coil embolization or an Amplatzer occlusion device (9).
Hayakawa et al. (10) and Madhusudhan et al. (11) performed transarterial embolization in patients with massive hemoptysis due to sequestration; they obtained a partial result with gelatin sponge alone and a complete result with a combination of fibrin sponge and sclerosing agents. Only two cases of therapeutic embolization by coils of a systemic arterialization of normal lung have been described in the literature (12). Preoperative arteriography with selective embolization reduces the risk of intraoperative bleeding; indeed, inadvertent injury to a systemic artery during lung resection can lead to massive hemorrhage. The problems are compounded by the friable nature of the artery, due to chronic inflammation. The artery may also develop atherosclerosis and become aneurysmal (13).
We suggest the possibility of performing a wedge resection instead of a lobectomy especially in young patients with small pulmonary sequestration as in the case presented. In fact the long life expectancy and the absence of neoplastic disease invites to follow the concept of “lung sparing” procedures. In our study, angioembolization was found to be useful in reducing the risk of intraoperative bleeding avoiding any unmanageable complications during surgical intervention such as sub-diaphragmatic abdominal bleeding. Embolization may also favour the thoracoscopic approach to solve this type of problem.
In conclusion thoracoscopic surgery has many benefits in expert hands: less postoperative pain, rapid return to daily activities and early discharge with low rate of complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
References
- Gonzalez D, Garcia J, Fieira E, et al. Video-assisted thoracoscopic lobectomy in the treatment of intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2011;12:77-9. [Crossref] [PubMed]
- Andrade CF, Ferreira HP, Fischer GB. Congenital lung malformations. J Bras Pneumol 2011;37:259-71. [Crossref] [PubMed]
- Savic B, Birtel FJ, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96-101. [Crossref] [PubMed]
- Pence K, Gaur P, Chan E, et al. Video-assisted thoracoscopic resection of intralobar pulmonary sequestration in adults. J Case Rep Images Surg 2016;2:39-42.
- Kestenholz PB, Schneiter D, Hillinger S, et al. Thoracoscopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg 2006;29:815-8. [Crossref] [PubMed]
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg 2011;40:e39-42. [Crossref] [PubMed]
- Leoncini G, Rossi UG, Ferro C, et al. Endovascular treatment of pulmonary sequestration in adults using Amplatzer® vascular plugs. Interact Cardiovasc Thorac Surg 2011;12:98-100. [Crossref] [PubMed]
- Irodi A, Prabhu SM, John RA, et al. Congenital bronchopulmonary vascular malformations, "sequestration" and beyond. Indian J Radiol Imaging 2015;25:35-43. [Crossref] [PubMed]
- Zener R, Bottoni D, Zaleski A, et al. Transarterial embolization of intralobar pulmonary sequestration in a young adult with hemoptysis. J Thorac Dis 2017;9:E188-93. [Crossref] [PubMed]
- Hayakawa K, Soga T, Hamamoto K, et al. Massive hemoptysis from a pulmonary sequestration controlled by embolization of aberrant pulmonary arteries: case report. Cardiovasc Intervent Radiol 1991;14:345-8. [Crossref] [PubMed]
- Madhusudhan KS, Das CJ, Dutta R, et al. Endovascular embolization of pulmonary sequestration in an adult. J Vasc Interv Radiol 2009;20:1640-2. [Crossref] [PubMed]
- Chabbert V, Doussau-Thuron S, Otal P, et al. Endovascular treatment of aberrant systemic arterial supply to normal basilar segments of the right lower lobe: case report and review of the literature. Cardiovasc Intervent Radiol 2002;25:212-5. [Crossref] [PubMed]
- Saxena P, Marshall M, Ng L, et al. Preoperative embolization of aberrant systemic artery in sequestration of lung. Asian Cardiovasc Thorac Ann 2011;19:357-9. [Crossref] [PubMed]
Cite this article as: Fabbri N, Tamburini N, Galeotti R, Quarantotto F, Maniscalco P, Rinaldi R, Salviato E, Cavallesco G. A rare case of intralobar pulmonary sequestration: combined endovascular and video-assisted thoracoscopic approach. AME Case Rep 2018;2:19.