Anaplastic thyroid carcinoma mimicking thyroid abscess
Case presentation
A 52-year-old Chinese male initially presented to the surgical clinic with an enlarging right neck mass of 1 month duration, with no compressive symptoms nor other clinical signs and symptoms to suggest a malignant diagnosis. Ultrasound revealed an enlarged right thyroid lobe with large cystic nodule measuring 2.8 cm × 2.3 cm × 5.2 cm with areas of calcification. Ultrasound guided fine needle aspiration revealed blood stained seropurulent fluid compatible with an abscess with no evidence of malignancy on cytology.
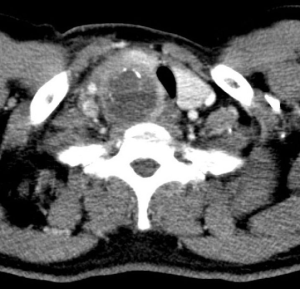
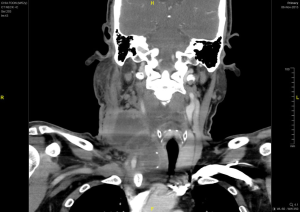
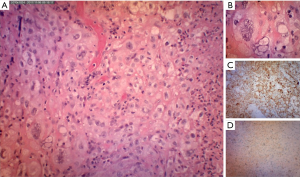
The neck swelling increased in size post aspiration with worsening pain and intermittent fever which was unresponsive to intravenous antibiotics and quick reaccumulation despite multiple aspiration attempts while warded. A contrasted computed tomography of the neck done showed a large right thyroid cyst measuring 2.6 cm × 2.3 cm × 5.2 cm with a small calcified nodule; causing a compressive effect causing tracheal deviation to left with tracheal narrowing. A multi-loculated rim enhancing collection was also seen at the right supraclavicular region extending superiorly to C5 level with largest locule measuring 3.7 cm × 3.8 cm with multiple enlarged right cervical lymph nodes seen (Figures 1,2). Patient was referred to the otorhinolaryngology department whereby an incision and drainage was performed. Twenty milliliters of blood stained seropurulent fluid was drained from the abscess cavity within the substance of the sternocleidomastoid muscle. Histopathology of enlarged cervical node noted during surgery reveal metastatic carcinoma and abscess wall was negative for malignancy. Further clinical examination did not reveal any other likely primary head and neck source of carcinoma apart from the thyroid gland. A decision was made to proceed with right hemithyroidectomy and right neck dissection. However, intra-operatively noted right thyroid lobe was hard and fixed to trachea, right internal jugular vein and the right carotid artery, with multiple enlarged cervical nodes. These findings were not present in the CT scan which was done 1 month before. Wedge biopsy of right thyroid lobe revealed a necrotic center with the presence of pus. Histopathology of right thyroid lobe biopsy was diagnostic of anaplastic carcinoma with focal squamous differentiation (Figure 3).
Discussion
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive malignancies with few patients surviving more than 6 months from initial presentation (1). ATC typically progresses rapidly with observed tumour doubling time as brief as 3 days (2). ATC constitutes less than 2% of all thyroid carcinomas and it is thought to arise from a terminal dedifferentiation of preexisting carcinomas of the thyroid follicular cell. This association has long been suggested from the consistent observation of coexisting follicular or papillary thyroid carcinoma (PTC) with ATC. This hypothesis has been further substantiated by modern genetic analysis, showing shared genetic alterations in the differentiated thyroid cancers and ATC found in the same patients (2).
Most patients with ATC typically present in the sixth or seventh decade of life. There is a slight female to male preponderance in the range of 1.5–2 (3). The hallmark of clinical presentation is a history of rapidly enlarging low anterior neck mass which is hard on palpation and fixed to underlying structures, often with symptoms of hoarseness, respiratory obstruction, dysphagia and neck pain. More than a third of patients present with sudden enlargement of a long-standing goiter. ATC is known for rapid local regional and metastatic spread. More than 80% have cervical lymph node metastasis at presentation and 20–50% have systemic metastasis (4,5).
Case reports of unusual presentations of ATC include bradycardia possibly from compression of vagus nerve, superior vena cava syndrome, thyrotoxicosis, acute Horner syndrome, leukocytosis from granulocyte colony-stimulating factor secretion and ball-valve type respiratory obstruction (2,6). The presentation of ATC in the form of thyroid cyst which was subsequently complicated with a neck abscess is highly unusual and no similar case could be found in the literature. The presence of thyroid cyst does not necessarily signify a benign lesion because PTC may manifest as cystic masses, and PTC may be present in 14% to 32% of all cystic nodules (7,8). Persistence of a cyst after multiple drainage attempts, quick re-accumulation of cyst, and red fluid on aspiration should raise suspicion for a carcinoma (9). We hypothesize that this patient could possibly have a long-standing PTC which was unnoticed until it has dedifferentiated into an ATC. However this could not be proven on histopathological exam as only wedge biopsy was taken. The ATC could also have arisen ‘de-novo’ with subsequent cyst formation due to tumour ischemia from rapid tumour growth causing tissue necrosis and liquefaction with subsequent secondary infection.
The diagnosis of ATC can often be established from fine needle aspiration cytology in most cases although a formal biopsy is occasionally necessary to exclude a lymphoma. The three predominant histologic patterns seen in ATC include spindle cell in 53%, giant cell in 50%, and squamoid in 19% with sometimes overlapping histologic types (2). Staging assessment with F-fluorodeoxyglucose positron emission tomography (FDG-PET) fused coincident computerized tomography scan (FDG-PET/CT) is uniquely valuable in ATC due to its enhanced expression of glucose transporter (GLUT-1), resulting in greater glucose uptake. Magnetic resonance imaging and CT scans are other alternatives for staging. Management of ATC is extremely difficult, requiring a multidisciplinary approach.
Surgical resection may be attempted in resectable disease or debulking may be performed for palliation. Tracheostomy needs careful consideration as tumour growth may progress at tracheostomy site with consequent hemorrhage into and around tracheostomy tube and difficult tracheostomy change. It is not advisable to perform tracheostomy except in patients whom imminent suffocation is likely, dyspnea unresponsive to corticosteroids and significant airway compromise undergoing chemoradiotherapy (2,10). Minor airway related issues can be overcome with use of humidified air, rest and occasional use of short term corticosteroids (11).
In summary, multimodality treatment is recommended for ATC with early surgery in resectable disease or debulking, followed by hyperfractionated radiotherapy and chemotherapy using doxorubicin. Most patients are incurable; however, a multimodality approach might improve local control and extend survival in selected individuals (12). Median survival is 2–6 months and survival beyond 2 years is 12% (13,14).
Acknowledgements
The authors want to thank Loh Aik Ban for the language editing assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Leeper RD. Thyroid cancer. Med Clin North Am 1985;69:1079-96. [Crossref] [PubMed]
- Ain KB, Valentino J. Anaplastic thyroid cancer and thyroid lymphoma. In: Randolph GW, editor. Surgery of the Thyroid and Parathyroid Glands. Philadelphia: Elsevier Saunders, 2003:242-3.
- Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005;103:1330-5. [Crossref] [PubMed]
- Demeter JG, De Jong SA, Lawrence AM, et al. Anaplastic thyroid carcinoma: risk factors and outcome. Surgery 1991;110:956-61; discussion 961-3. [PubMed]
- O'Neill JP, Shaha AR. Anaplastic thyroid cancer. Oral Oncol 2013;49:702-6. [Crossref] [PubMed]
- Villa ML, Mukherjee JJ, Tran NQ, et al. Anaplastic thyroid carcinoma with destructive thyrotoxicosis in a patient with preexisting multinodular goiter. Thyroid 2004;14:227-30. [Crossref] [PubMed]
- de los Santos ET, Keyhani-Rofagha S, Cunningham JJ, et al. Cystic thyroid nodules. The dilemma of malignant lesions. Arch Intern Med 1990;150:1422-7. [Crossref] [PubMed]
- Rosen IB, Provias JP, Walfish PG. Pathologic nature of cystic thyroid nodules selected for surgery by needle aspiration biopsy. Surgery 1986;100:606-13. [PubMed]
- Sadler GP, Clark OH, van Heerdeen JA, et al. Thyroid and parathyroid. In: Schwartz SI, Shires GT, Spencer FC, et al. editors. Principles of Surgery. New York: McGraw-Hill, 1999:1661-713.
- Shaha AR, Ferlito A, Owen RP, et al. Airway issues in anaplastic thyroid carcinoma. Eur Arch Otorhinolaryngol 2013;270:2579-83. [Crossref] [PubMed]
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104-39. [Crossref] [PubMed]
- Veness MJ, Porter GS, Morgan GJ. Anaplastic thyroid carcinoma: dismal outcome despite current treatment approach. ANZ J Surg 2004;74:559-62. [Crossref] [PubMed]
- Kim JH, Leeper RD. Treatment of locally advanced thyroid carcinoma with combination doxorubicin and radiation therapy. Cancer 1987;60:2372-5. [Crossref] [PubMed]
- Tennvall J, Lundell G, Hallquist A, et al. Combined doxorubicin, hyperfractionated radiotherapy, and surgery in anaplastic thyroid carcinoma. Report on two protocols. The Swedish Anaplastic Thyroid Cancer Group. Cancer 1994;74:1348-54. [Crossref] [PubMed]
Cite this article as: Loh TL, Zulkiflee AB. Anaplastic thyroid carcinoma mimicking thyroid abscess. AME Case Rep 2018;2:20.