Diagnosis and management of spinal metastasis of primary brain tumours
Introduction
The prognosis for patients with high grade glioma is poor. Despite aggressive multimodal treatment including surgical resection, radiotherapy and chemotherapy, the natural clinical course is tumour relapse. Median survival is less than 2 years (1-3). About 90% of these lesions recur intracranially. In contrast, the frequency of spinal cord disease is less than 2% (4,5). In daily practice, symptomatic spinal cord metastases and leptomeningeal disease in high-grade gliomas are rare events. In the literature, there are less than a hundred cases of symptomatic spinal cord or leptomeningeal metastasis reported (3,6). We report two cases of symptomatic spinal cord metastases from high-grade glioma. One of the learning points we want to share is to think in the possibility of spinal cord metastases from brain gliomas.
Case report
Case 1
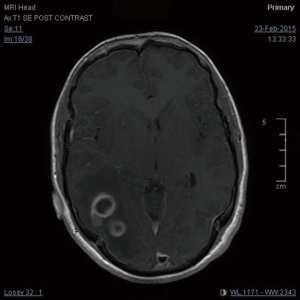
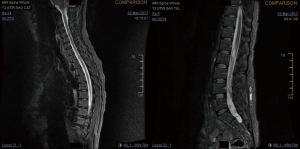
A 27-year-old gentleman presented with a 5-month history of tinnitus and increasing headaches in the occipital and orbital area. A CT scan was performed showing an intracerebral mass. An MRI head (Figure 1) was subsequently performed showing appearances consistent with a high-grade astroglial tumour with multifocal non-enhancing T2 hyperintense regions suggesting de-differentiation on a background of multifocal low-grade disease. Subtotal resection was performed and carmustine wafers were added to the surgical bed. Pathological tissue diagnosis was consistent with a right temporal lobe glioblastoma multiforme (GBM), IDH1 wild-type. The patient was treated with concurrent chemo-radiotherapy. In brief, intensity-modulated radiotherapy (IMRT) up to 60 Gy in 30 fractions to the right temporal lobe surgical bed with a margin and concurrent temozolomide (75 mg/m2/d × 7 d/week for 42 days). Five cycles of adjuvant temozolomide was given after chemo-radiation. The patient remained PS 0–1 during the treatment. Three weeks after finishing adjuvant temozolomide, the patient was admitted presenting left lower back pain, left lower limb heaviness and right lower limb sensory loss and numbness. At the time of admission neurological examination was normal except for sensory loss with a slightly impaired feeling in the lower right limb. The MRI of the brain showed mixed appearances, with response in the surgical bed but new foci in the corpus callosum and anterior periventricular region of the right hemisphere. The case was discussed at the Neuro Oncology Multidisciplinar Team meeting and advised to start second-line treatment with PCV (procarbazine, lomustine, and vincristine) was recommended. During this time, the patient had progressive neurological symptoms worsening with new leg weakness, unsteadiness, constipation, and difficulty passing urine. A spinal MRI (Figure 2) was performed showing multiple leptomeningeal and drop spinal metastases. The patient was treated with dexamethasone and underwent palliative radiation therapy to the thoracic and lumbosacral spine (from T12 to S3, 30 Gy in 10 fractions and from T5–T9, 20 Gy in 5 fractions). His clinical status continued to deteriorate declining and he was transferred to a palliative care service.
Case 2
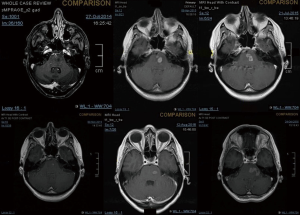
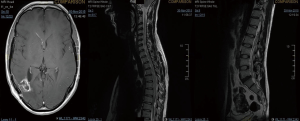
A 43-year-old lady with a past medical history of von Willebrand’s disease, had problems with double vision, nausea and headaches. An MRI of the brain (Figure 3) was performed showing a likely pontine low-grade glioma. Due her past medical history of von Willebrand’s disease and the location of the lesion a biopsy was not possible. The patient was treated with radiotherapy up to 54 Gy in 30 fractions to the visible tumour on MRI. The first follow-up MRI done 2 months after radiotherapy showed an increase in the size of the cystic component of the tumour with new multifocal solid nodular enhancement. Concluding remark stated that the MRI features most likely represented disease progression rather than radiotherapy-related changes. At the same time, the patient had been getting increasing problems with her vision in her left eye, weakness and numbness in her right side and has had a few falls. Neurological examination showed grade 3/5 power loss in her right arm and 4+/5 in her right leg. Because of the behaviour of high-grade glioma, salvage chemotherapy with temozolomide, six cycles every 28 days was planned. She started with dexamethasone as symptomatic treatment as well. During chemotherapy, the patient’s symptoms improved and allowed reduction of the steroids reduction. She completed six cycles of temozolomide and started follow-up with MRI. Eleven months after finishing salvage chemotherapy progressive disease was noted with a new enhancing lesion in the right posterior cerebellum and increase in size and enhancement of the left pontine lesion. Simultaneously, the patient started with slight right leg weakness and headaches at the right side. She started PCV as second-line chemotherapy. Unfortunately, PCV was stopped after two cycles due to neutropenic sepsis and worsening performance status. Six wee4ks later the patient was admitted to hospital with left leg weakness and urinary retention. A spinal MRI (Figure 4) was done, showing multiple cystic and solid intradural lesions in the lumbar spine causing nerve cauda equine compression. The patient was treated with dexamethasone and underwent palliative radiation therapy (8 Gy in single fraction) to the lumbar spine (T12–S5). She was transferred to a palliative care service.
Discussion
Choucair et al. reviewed in 1980s 1,047 patients who had an original diagnosis of supratentorial GBM or other anaplastic glioma to determine the percentage of patients who developed multiple central nervous system gliomas. Only 1.2% to 1.5% of all the patients developed multiple metastasis in central nervous system (4). There have been 64 reported patients with symptomatic spinal dissemination of intracranial high-grade glioma including those discussed in this paper. Clinical symptoms are due to the compression of the spinal cord or cauda equina. The most common sites of spinal metastases are the lower thoracic, upper lumbar, and lumbosacral regions, cauda equina and thecal sac (7,8). Most frequent neurological symptoms were back pain, paraparesis and sensory level depending on the affected spinal segment, radicular pain and autonomic dysfunction like bladder and/or bowel incontinence according to the literature (9). Our patients had back pain, paraparesis and different levels of autonomic dysfunction. The diagnosis should be made today with enhanced MRI when spinal metastasis is suspected. Whole spine MRI should be requested because, as we illustrated with our cases, several levels could be involved at the same time.
The mechanisms of tumour spread along the central nervous system include perivascular growth; local invasion (choroid plexus, cortical surface and subpial space); and along white matter tracts associated with craniotomies and location of the tumours adjacent or proximal to ventricular structures (6,9,10). In our cases, both were adjacent to ventricular structures at relapse (Figures 2,3F).
Therapeutic interventions for disseminated spinal metastasis of high-grade gliomas include steroids, decompressive surgery, radiotherapy and chemotherapy. Due to the poor survival, once multiple central nervous system metastasis is diagnosed, steroids and palliative radiotherapy are usually used to preserve quality of life.
Further studies are needed to find predictive factors for developing multiple metastasis in the central nervous system. Craniospinal irradiation may be a suitable therapeutic option for these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent is unavailable because both patients had passed away before our literature review.
References
- Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 1993;85:704-10. [Crossref] [PubMed]
- Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 2002;20:1375-82. [Crossref] [PubMed]
- Lawton CD, Nagasawa DT, Yang I, et al. Leptomeningeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of disease. J Neurosurg Spine 2012;17:438-48. [Crossref] [PubMed]
- Choucair AK, Levin VA, Gutin PH, et al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg 1986;65:654-8. [Crossref] [PubMed]
- Schuster H, Jellinger K, Gund A, et al. Extracranial metastases of anaplastic cerebral gliomas. Acta Neurochir (Wien) 1976;35:247-59. [Crossref] [PubMed]
- Shahideh M, Fallah A, Munoz DG, et al. Systematic review of primary intracranial glioblastoma multiforme with symptomatic spinal metastases, with two illustrative patients. J Clin Neurosci 2012;19:1080-6. [Crossref] [PubMed]
- Lam CH, Cosgrove GR, Drislane FW, et al. Spinal leptomeningeal metastasis from cerebral glioblastoma. Appearance on magnetic resonance imaging. Surg Neurol 1991;35:377-80. [Crossref] [PubMed]
- Birbilis TA, Matis GK, Eleftheriadis SG, et al. Spinal metastasis of glioblastoma multiforme: an uncommon suspect? Spine (Phila Pa 1976) 2010;35:E264-9. [Crossref] [PubMed]
- Tinchon A, Oberndorfer S, Marosi C, et al. Malignant spinal cord compression in cerebral glioblastoma multiforme: a multicenter case series and review of the literature. J Neurooncol 2012;110:221-6. [Crossref] [PubMed]
- Tai P, Dubey A, Salim M, et al. Diagnosis and Management of Spinal Metastasis of Glioblastoma. Can J Neurol Sci 2015;42:410-3. [Crossref] [PubMed]
Cite this article as: Chen-Zhao X, Aznar-García L. Diagnosis and management of spinal metastasis of primary brain tumours. AME Case Rep 2018;2:26.