Surgical management of an adult manifestation of Ewing sarcoma of the spine—a case report
Introduction
Ewing sarcoma (ES) is a rare malignant bone tumor. It is a subtype of a group of malignant tumors called primitive neuroectodermal tumors (PNET) (1). Pathologically, ES shows sheets of small round blue neoplastic cells found in bones or in the surrounding soft tissues. It is more common in males and usually presents in childhood or early adulthood, with a peak incidence between 10 and 20 years of age (2). The most common sites of the disease are the pelvis and the femur (3). Its occurrence in axial skeleton, to the lesser extent in the mobile spine as a non-metastatic tumor is extremely rare. The prognosis in ES of the spine with presenting neurological deficit is mostly poor (4). ES could be divided in regard of the outcome in sacral and non-sacral ES, with the sacral type being more aggressive, because it is less responsive to treatment. There is no specific presentation or symptom of the ES, leaving the definite diagnosis for the tissue biopsy. Treatment of the local disease which presented in this case is primarily through initial neoadjuvant or induction chemotherapy followed by local treatment including surgery and/or radiation (5).
To our knowledge, there is only a small amount of reported cases of primary ES of the spine in adult patients. They are all sporadically reported, with varying success. Because of the low incidence of the disease, there are low evidence-based guidelines regarding local disease therapy that outline their management.
In this article, we describe our successful experience with an atypical case of an ES of an adult thoracic spine presenting with neurological deficits and review the current literature on the clinical characteristics, diagnosis, and management of this rare condition.
Case presentation
History of presentation
A 56-year-old female presented with acute incomplete paraparesis. One month prior to presentation she had an increasing upper thoracic back pain without history of back pain. Later she developed an incomplete paraparesis (ASIA B) and sensory deficit sub Th2 with spinal ataxia and coordination disorder in the lower extremities and she referred to our emergency ambulance for urgent treatment.
Past medical history
She was a known case of breast CA 5 years prior to presentation (which was already treated with radio-chemotherapy and surgically).
Diagnostic work up
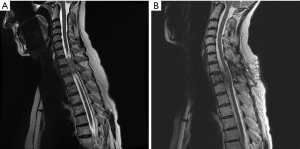
MRI showed a mass lesion in the Th2 vertebra with spinal cord compression. A tumor metastasis was suspected by known case of breast CA in the upper thoracic spine, as shown in Figure 1A.
Emergent treatment
Immediately after diagnosis and preparation, an emergency debulking of the tumor was performed and the spinal canal was meticulously decompressed (Figure 1B). Postoperatively, the neurological deficits slowly recovered (Grade C in ASIA Score).
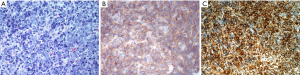
A re-staging diagnosis (with a CT scan of the lungs and a bone scan and mammography) was done but without revealing a focus of a primary tumor. The histological findings showed an ES unexpectedly. The histological results can be seen in Figure 2.
Course of the treatment
After obtaining the histopathological diagnosis she received chemotherapy (6 cycles VIDE Vincristine, Ifosfamide, Doxorubicin, Etoposide). MRI obtained 6 months after the first operation showed a complete resection of the extraosseous tumor fraction (Figure 1B). However, an additional infiltration of the first thoracic vertebra was suspected, therefor an en bloc-resection of the 1st and 2nd thoracic vertebra was recommended by the interdisciplinary tumor board.
Surgery
The procedure was performed via a combined dorsal and ventral approach, with dorsal stabilization by transpedicular C6/C7–Th4/5 fixation and ventral vertebral bodies Th1 and Th2 replacement along with anterior plating C6–Th4 .
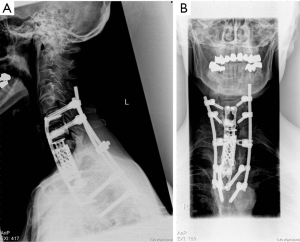
In the first step, a posterior instrumentation from the 6th cervical vertebra to the 4th thoracic vertebra was performed with the aid of neuronavigational guidance. After changing the patient position from prone to supine, en bloc excision was performed via a left-sided anterolateral approach. The vertebral body reconstruction was performed by means of an expandable vertebral body replacement and plating. In addition, nanocristal hydroxyl apatite (Nanobone® SpongioTech, Weida, Germany) was added as a fusion material. The resection was aided by ultrasonic bone scalpel (Söring, Quickborn, Germany) and the entire procedure was performed with continuous neurophysiological monitoring (MAP, SSEP). Figure 3 shows the X-rays taken after stabilization.
Postoperatively, the patient was stable and in the recovering phase with low grade proximal paraparesis. She was sent to rehabilitation. A planned follow up 3, 6, 12 and 18 months after the en bloc resection was done and revealed no recurrence.
At 18 months follow up, she remained free of disease and experienced complete resolution of her back pain, radiculopathy, and was able to walk free without crutches (AISA Grade E).
Discussion
We present our experience in managing a case of ES of the thoracic spine in an adult female and reviewing the advantages of an en-bloc excision of the local tumor.
The diagnosis usually takes couple of weeks to months, while definitive diagnosis requires tissue biopsy. The main reasons of the delayed diagnosis in adults are a low clinical suspicion of the disease and the gradual onset of nonspecific symptoms such as localized pain or swelling over few weeks or sometimes months (6,7), with or without constitutional symptoms. This patient had symptoms due to the space-occupying lesion causing pressure effect with spinal cord compression with initial nonspecific back pain.
The goal of the initial evaluation after the diagnosis is to evaluate local disease extent and the metastatic spread. The current standard of care involves chemotherapy and local disease control with surgery or radiation regardless of the extent of disease at presentation. The suggested neoadjuvant treatment regimens in adults are considered just the same as in children (8). The duration of the treatment lasts usually 6–9 months and consists of alternating courses of two chemotherapeutic regimens: (I) vincristine, doxorubicin, and cyclophosphamide and (II) ifosfamide and etoposide (9).
Local disease control can be achieved by surgery and/or radiation therapy. We noticed a huge advantage for the en bloc excision in our case versus intralesional excision in non-metastatic disease with the aid of ultrasonic scalpel to prevent soft tissue damage and therefore opting for a most radical excision (10). Previous retrospective studies also showed that adequate surgical resection had a positive impact on the survival rate regarding ES of the spine (11). Data that document the effectiveness of the current approach in the adult spine are still limited (12).
In our case, the patient was treated with tumor debulking surgery followed by chemotherapy after the definitive diagnosis of ES and an en bloc resection of the remaining tumor during a second stage.
In comparison to ES in children we saw in previous studies that the age would be not significant regarding the treatment and prognosis if the right diagnosis was made at the right time, which is usually not the case in adults.
Age as prognostic factor is still controversial. Some studies showed that the age could be a bad prognostic factor (3,13) and others showed no statistically significant difference (14).
The two most significant factors currently known to determine the prognosis in patients with ES are the presence or absence of metastatic disease and the primary site of the tumor, with axial-skeleton presentation being less favorable than those with distal extremities (4,15,16). One of the main reasons why ES of the spine is considered as bad prognostic factor is the difficulty in achieving safe resection margins. Our case, dealing with uncommon disease in an atypical location with bad prognostic factors (by affecting the spine with presenting neurological deficits) was challenging.
The en bloc resection with possible marginal margins, as well as a favorable initial response to induction therapy had an important impact on the prognosis. The surgical management could also alleviate the spinal pain as well as improving the neurological deficits.
Further prospective studies exploring other treatment options and their long-term effects in adult spinal ES are required to tailor better evidence-based treatment guidelines.
Conclusions
This case highlights the management of an ES of an adult spine and the advantages of en bloc resection of the local disease. We highlight the advantage of a complete resection of the local tumor to achieving a better prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: JU Müller is an unpaid consultant for Söring GmbH, and has received honoraria for lectures/workshops. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient to publish this manuscript and any accompanying images.
References
- Ambros IM, Ambros PF, Strehl S, et al. MIC2 is a specific marker for ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer 1991;67:1886-93. [Crossref] [PubMed]
- Hense HW, Ahrens S, Paulussen M, et al. Descriptive epidemiology of Ewing’s tumor--analysis of German patients from (EI) CESS 1980-1997. Klinische Padiatrie 1999;211:271-5. [Crossref] [PubMed]
- Verma V, Denniston KA, Lin CJ, et al. A Comparison of Pediatric vs. Adult Patients with the Ewing Sarcoma Family of Tumors. Front Oncol 2017;7:82. [Crossref] [PubMed]
- Cash T, McIlvaine E, Krailo MD, et al. Comparison of clinical features and outcomes in patients with extraskeletal versus skeletal localized Ewing sarcoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer 2016;63:1771-9. [Crossref] [PubMed]
- Mirzaei L, Kaal SEJ, Schreuder HWB, et al. The Neurological Compromised Spine Due to Ewing Sarcoma. What First: Surgery or Chemotherapy? Therapy, Survival, and Neurological Outcome of 15 Cases With Primary Ewing Sarcoma of the Vertebral Column. Neurosurgery 2015;77:718-24; discussion 724-5. [Crossref] [PubMed]
- Rud NP, Reiman HM, Pritchard DJ, et al. Extraosseous Ewing’s sarcoma. A study of 42 cases. Cancer 1989;64:1548-53. [Crossref] [PubMed]
- Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am 2000;82:667-74. [Crossref] [PubMed]
- Ahmed SK, Robinson SI, Okuno SH, et al. Adult ewing sarcoma: survival and local control outcomes in 102 patients with localized disease. Sarcoma 2013;2013. [Crossref] [PubMed]
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694-701. [Crossref] [PubMed]
- Hu X, Ohnmeiss DD, Lieberman IH. Use of an ultrasonic osteotome device in spine surgery: experience from the first 128 patients. Eur Spine J 2013;22:2845-9. [Crossref] [PubMed]
- Sciubba DM, De la Garza Ramos R, Goodwin CR, et al. Clinical, surgical, and molecular prognostic factors for survival after spinal sarcoma resection. Neurosurg Focus 2016;41. [Crossref] [PubMed]
- Sewell MD, Tan KA, Quraishi NA, et al. Systematic Review of En Bloc Resection in the Management of Ewing’s Sarcoma of the Mobile Spine with Respect to Local Control and Disease-Free Survival. Medicine 2015;94. [Crossref] [PubMed]
- Baldini EH, Demetri GD, Fletcher CDM, et al. Adults With Ewing’s Sarcoma/Primitive Neuroectodermal Tumor: Adverse Effect of Older Age and Primary Extraosseous Disease on Outcome. Ann Surg 1999;230:79-86. [Crossref] [PubMed]
- Valdes M, Nicholas G, Verma S, et al. Systemic Therapy Outcomes in Adult Patients with Ewing Sarcoma Family of Tumors. Case Rep Oncol 2017;10:462-72. [Crossref] [PubMed]
- Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol 2000;18:3108-14. [Crossref] [PubMed]
- Paulussen M, Ahrens S, Dunst J, et al. Localized Ewing Tumor of Bone: Final Results of the Cooperative Ewing’s Sarcoma Study CESS 86. J Clin Oncol 2001;19:1818-29. [Crossref] [PubMed]
Cite this article as: Shoubash L, Nowak S, Vogelgesang S, Schroeder HW, Müller JU. Surgical management of an adult manifestation of Ewing sarcoma of the spine—a case report. AME Case Rep 2018;2:34.


