Deciduoid type malignant pleural mesothelioma: a case report
Introduction
Deciduoid type malignant mesothelioma (MM) has been reported as a rare subtype of epithelioid mesothelioma that pathologically mimics decidua and is associated with poor prognosis (1,2). The clinical and pathological futures of deciduoid MM are unknown. Here, we present a case of deciduoid type malignant pleural mesothelioma (MPM) which has progressed aggressively.
Case presentation
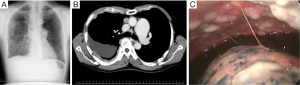
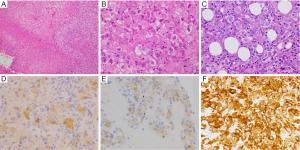
A 55-year-old man who might have been exposed to asbestos a few decades ago had severe right back pain for two weeks and came to our hospital. Chest X-ray scanning (Figure 1A) and computed tomography (CT) (Figure 1B) revealed pleural effusion, with pleural thickness on his right thoracic space, without a lung nodule. Pleural fluid cytology showed atypical cells suggested, but not concluded for malignancy. The hyaluronic acid concentration in the pleural fluid was 36,400 ng/mL. A blood test showed soluble mesothelin level was slightly high (1.5 nmol/L), while both CEA and CYFRA levels were within normal range. We suspected a diagnosis of right MPM, and right pleural biopsy was performed. The intraoperative findings showed disseminated white nodules on the parietal pleura (Figure 1C). The pathological findings showed polygonal cells with abundant eosinophilic cytoplasm that were arranged in solid nests, which mimic decidua (Figure 2A,B) and invade the fatty tissue (Figure 2C). Deciduoid type morphology was predominantly seen in whole tissue. The mitotic figures were 13/10 HPF. The immunohistochemical (IHC) staining revealed that the tumor cells were positive for calretinin (Figure 2D), D2-40 (Figure 2E), IMP3 (Figure 2F), GLUT-1, and CAM5.2, and negative for BerEP4, CEA, desmin, TTF1, and CD146. From these findings, the pleural nodules were pathologically diagnosed as deciduoid type MPM; clinical T3N1M0 stage IIIA (3-5). After the pleural biopsy, both the area and level of his back pain worsened, day after day. Neither oral nor transdermal opioids were able to control his back pain, while epidural anesthesia showed an effect. Although he received two cycles of standard chemotherapy with cisplatin combined with pemetrexed, his disease showed rapid progression and he died within 2 months after MPM diagnosis.
Discussion
Deciduoid type MM was first reported in 1985 by Talerman et al. as the case of a 13-year-old girl with malignant peritoneum mesothelioma (1). In the beginning, all reported cases of deciduoid type MM were located in the peritoneum or were paratesticular (1,6,7). In 2000, however, cases of deciduoid type MPM were reported from two groups (8,9). Clinical characteristics of deciduoid type MM were reviewed by Ordonez and defined as a variant of pleomorphic MM, for which nuclear grade could predict prognosis. The present patient showed a high mitotic count (13/10 HPF) and was diagnosed with high grade deciduoid mesothelioma, which has been reported as a poor prognostic variant (2). The IHC was also summarized by Ordonez, who showed that all tested cases were positive for calretinin, pan-keratin, keratin 5/6, keratin 8 (CAM5.2), mesothelin, and HBME-1 (2). More recently, Paliogiannis et al. comprehensively reviewed previous literatures and reported that immunostaining for calretinin was positive in all tested cases (10). In line with previous reports (2), present patient was both calretinin and CAM5.2 positive. However, the current diagnostic markers for deciduoid type MPM are immature, and national wide studies that construct IHC panels for rare tumors, such as deciduoid type mesothelioma, are required.
In this patient, the disease progression was quite aggressive, despite receiving standard chemotherapy, which is why we focused on the role of chemotherapy in patients with deciduoid type MPM. Our review of the literature including present case evidenced only 6 cases of deciduoid type MPM which were treated with chemotherapy alone (9,11) (Table 1). The cases with moderate mitotic count (2–5/10 HPF) survived more than 1 year, while the cases with high mitotic count (>5/10 HPF) dead within 4 months, suggested that mitotic count is a promising predictive factor for survival of deciduoid type mesothelioma. In present case, tumor strongly expressed IPM3, supported recent report showing that IMP3 expression was a useful prognostic biomarker in patients with malignant peritoneal mesothelioma (12), although there is no information of IMP3 status in other 5 cases. Unfortunately, the effect of chemotherapy could not be available in other 5 cases, although it is also very important to know the chemo-sensitivity of this variant.

Full table
Recent clinical studies demonstrated that immunocheckpoint targeting therapy showed good responses in patient with MPM, regardless of pathological subtype (epithelial or sarcomatoid) (13). To improve the clinical outcomes of rare tumors, including variant type MPM, prospective registration studies should be conducted in a nation- or worldwide settings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Talerman A, Montero JR, Chilcote RR, et al. Diffuse malignant peritoneal mesothelioma in a 13-year-old girl. Report of a case and review of the literature. Am J Surg Pathol 1985;9:73-80. [Crossref] [PubMed]
- Ordonez NG. Deciduoid mesothelioma: report of 21 cases with review of the literature. Mod Pathol 2012;25:1481-95. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Nascimento AG, Keeney GL, Fletcher CD. Deciduoid peritoneal mesothelioma. An unusual phenotype affecting young females. Am J Surg Pathol 1994;18:439-45. [Crossref] [PubMed]
- Orosz Z, Nagy P, Szentirmay Z, et al. Epithelial mesothelioma with deciduoid features. Virchows Arch 1999;434:263-6. [Crossref] [PubMed]
- Shanks JH, Harris M, Banerjee SS, et al. Mesotheliomas with deciduoid morphology: a morphologic spectrum and a variant not confined to young females. Am J Surg Pathol 2000;24:285-94. [Crossref] [PubMed]
- Ordonez NG. Epithelial mesothelioma with deciduoid features: report of four cases. Am J Surg Pathol 2000;24:816-23. [Crossref] [PubMed]
- Paliogiannis P, Putzu C, Ginesu GC, et al. Deciduoid mesothelioma of the thorax: A comprehensive review of the scientific literature. Clin Respir J 2018;12:848-56. [Crossref] [PubMed]
- Scattone A, Pennella A, Gentile M, et al. Comparative genomic hybridisation in malignant deciduoid mesothelioma. J Clin Pathol 2006;59:764-9. [Crossref] [PubMed]
- Hui S, Guo-Qi Z, Xiao-Zhong G, et al. IMP3 as a prognostic biomarker in patients with malignant peritoneal mesothelioma. Hum Pathol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
Cite this article as: Okita R, Nojima Y, Saisho S, Shimizu K, Shirai R, Kanomata N, Oka M, Nakata M. Deciduoid type malignant pleural mesothelioma: a case report. AME Case Rep 2018;2:43.


