A case report of sphenoid sinusitis causing opsoclonus myoclonus syndrome
Introduction
Opsoclonus myoclonus syndrome (OMS) is a rare neurological condition characterized by fast, multidirectional involuntary eye movements with the presence of myoclonus. The eye movements can be triggered by fixation on objects, whereas myoclonus is thought to be triggered by movement and commonly involves the limbs, face and trunk. In adults, OMS is associated with paraneoplastic, para-infectious and idiopathic causes (1). Here we describe an extremely rare aetiology of OMS alongside an overview of the condition.
Case presentation
A 26-year-old lady who was 19 weeks pregnant presented to her local obstetric service feeling generally weak and lethargic over the previous 6 days. She had been complaining of an intermittent headache in the preceding two weeks associated with one episode of vomiting.
She had particularly noticed weakness and a jittering sensation in her legs prior to them giving way whilst walking. This necessitated her having to lie down to rest and then requiring support to continue walking. Involuntary leg movements were seen on examination.
On admission she was noted to be tachycardic and pyrexial. CRP and WCC were normal on admission and she displayed no clinical signs of meningitis. She was commenced on intravenous antibiotics. Two days following admission she reported her eyes shaking whilst trying to focus on objects.
Examination revealed fluctuating amplitude of multidirectional ocular movements whilst trying to fixate on objects. Cranial nerve and fundoscopic examination were normal. Power, tone, reflexes and sensation were normal but it was queried whether she had some dysdiadochokinesis. The characteristic eye movements (opsoclonus) and involuntary leg movements (myoclonus) whilst walking led to a clinical diagnosis of OMS.
Investigations
The impression at this point was that of OMS and therefore magnetic resonance imaging (MRI) and lumbar puncture (LP) were requested. LP revealed clear cerebrospinal fluid (CSF) with 10/uL white blood cells, no organisms on microscopy and no viruses seen on PCR virology. The CSF was examined for the presence anti-Hu, -Yo and -Ri auto-antibodies which were not found. Serum voltage gated potassium channel antibodies were negative.
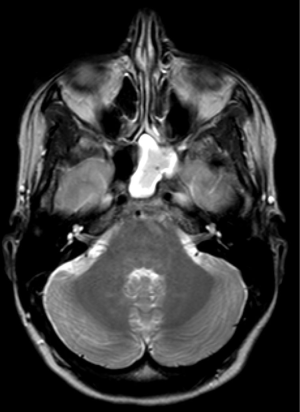
An MRI revealed a normal brain but noted there to be a left sphenoidal sinus empyema (Figure 1).
Given this finding, the ENT team were asked to review the patient. A flexible nasendoscopy revealed a deviated nasal septum to the left and frank pus on the left side with no clear view of the left ethmoidal air cells. A swab of the pus was sent for microscopy, culture and sensitivity. This pus swab yielded no growth of organisms.
Treatment
Antibiotics (ceftriaxone 2 grams daily and metronidazole 500 mg TDS), nasal decongestants and saline nasal douches were commenced.
Outcome
Ten days after the commencement of her symptoms and four days after admission the patient noticed a foul-tasting post-nasal drip suggesting spontaneous drainage of her empyema. Given her pregnancy, the decision to surgically open the sinus was delayed.
Both her opsoclonus and myoclonus resolved with treatment with antibiotics, decongestants and spontaneous drainage of the empyema. No bacteria were cultured from her CSF, nor peripheral blood cultures. The patient went on to make a full recovery, which was noted at one-month follow up. Given the patient was still pregnant, a decision was made not repeat the imaging.
Discussion
Complications from sphenoid sinusitis are uncommon. However, given the close proximity of the sphenoid sinus to the orbits, dura, and cranial nerves III, IV, V and VI; complications such as orbital cellulitis, meningitis and intracranial abscesses can be seen.
Assessment of the eyes and their movements in this case is key to the diagnosis. Saccadic intrusions are fast simultaneous jerky movements of the eyes that disrupt the normal process of eye fixation. They can be found in normal patients or be indicative of neurological pathologies. A latent period normally exists between the saccades termed the inter-saccadic interval. Identification or absence of the inter-saccadic interval helps to categorise saccadic intrusions and aids diagnosis for pathological causes.
Opsoclonus is a saccadic intrusion represented by fluctuating amplitudes of uncoordinated multidirectional saccades without an inter-saccadic interval. The presence of multidirectional saccades distinguishes it from ocular flutter, which usually has saccades in one plane only (2).
Myoclonus refers to involuntary muscle twitching, which in conjunction with opsoclonus has been described as OMS. The random eye and limb movements of OMS are thought to be due to cerebellar dysfunction and the syndrome has also been named the “dancing eyes, dancing feet syndrome” (3).
OMS can affect both adults and children and can be caused by para-neoplastic and para-infectious disease processes but there are also a number of cases that are idiopathic in nature. In children, there are strong associations with neuroblastoma and in adults, breast and lung carcinomas (3).
Several theories have tried to explain how malignancy or infections not directly involved with the central nervous system can cause such a syndrome. Circulating antibodies in serum and CSF are thought to act as part of an autoimmune mediated process by binding to cerebellar cells and directly affect their functioning (3), although antibodies are not identified in all cases.
A brainstem theory suggests damage or dis-inhibition of burst and omnipause cells in the brainstem that normally interact to produce saccadic eye movements (4). A cerebellar theory suggests there is dis-inhibition of the cerebellar fastigial nucleus (5).
Sahu and Prasad (1) suggest a thorough history, examination and some important investigations to ascertain an underlying aetiology given the syndrome’s associations with serious infections and occult malignancy. Initial investigations should include an MRI of the brain, CSF analysis and an electroencephalogram (EEG). The symptoms of OMS usually precede a diagnosis of a neoplasm when this is the aetiology, therefore it is suggested that clinicians actively seek to exclude such a cause. Hence, patients should undergo a CT scan of the chest, abdomen and pelvis and mammography with further consideration for the study of onconeural autoantibodies and positron emission tomography (PET)-CT scans (1).
However, in this case given the resolution of the syndrome and the need to limit radiation exposure to the developing foetus, a decision was made to not perform CT imaging.
The management is aimed at treating the underlying cause if identified, alongside immunotherapy; although no guidelines exist. For patients with a neoplastic cause for OMS, removal of the causative tumour has led to a partial, if not full neurological recovery in a recent comparative retrospective analysis (6).
Spontaneous empyema drainage and IV antibiotics improved the clinical picture for this patient. Alternatively, immunosuppressive therapies such as immunoglobulins, plasmapheresis, steroids and adrenocorticotropic hormone (ACTH) have been used (3).
In this case it was thought that the infectious process and not the direct extension of the sphenoidal sinus empyema was the trigger for the OMS. We found no evidence of any circulating autoantibodies. To our knowledge, sinusitis triggering OMS has not been described in the literature previously. Neither has OMS been described in pregnancy before. This woman’s pregnancy may have caused an element of immunosuppression and hence a limited inflammatory response to her empyema originally.
Conclusions
OMS is a rare neurological condition necessitating a through work-up for the presence of an underlying para-infectious or para-neoplastic cause. Sphenoid sinusitis has not been described previously in the literature as a cause for OMS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Sahu JK, Prasad K. The opsoclonus–myoclonus syndrome. Pract Neurol 2011;11:160-6. [Crossref] [PubMed]
- Lemos J, Eggenberger E. Saccadic intrusions: review and update. Curr Opin Neurol 2013;26:59-66. [Crossref] [PubMed]
- Scarff JR, Iftikhar B, Tatugade A, et al. Opsoclonus myoclonus. Innov Clin Neurosci 2011;8:29-31. [PubMed]
- Pranzatelli MR. The neurobiology of the opsoclonus-myoclonus syndrome. Clin Neuropharmacol 1992;15:186-228. [Crossref] [PubMed]
- Helmchen C, Rambold H, Sprenger A, et al. Cerebellar activation in opsoclonus: an fMRI study. Neurology 2003;61:412-5. [Crossref] [PubMed]
- Bataller L, Graus F, Saiz A, et al. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain 2001;124:437-43. [Crossref] [PubMed]
Cite this article as: Turner H, Snelling J, Martinez-Devesa P. A case report of sphenoid sinusitis causing opsoclonus myoclonus syndrome. AME Case Rep 2018;2:47.