
Late implant migration with neurologic compromise as a complication of scoliosis surgery
Introduction
Late neurological deficit following scoliosis surgery has been reported in very few patients in the English-language literature. The first report of late paraparesis (due to bony overgrowth) was from Eismont and Simeone in 1981 (1). In 1982, Court-Brown and McMaster (2) reported another late paraparesis presentation after posterior spinal fusion for scoliosis, this time due to a pseudarthrosis and fracture of a Harrington rod. Savini et al. in 1990 (3) also reported on three cases of late paraparesis: (I) two patients with progression of deformity after a spinal fusion; and (II) one patient with bony overgrowth following a pseudarthrosis. Previous to Savini, Roy et al. (4) had reported a case of late neurological deficit also following a non-union. Although there is no clear evidence to change the standard follow-up for postoperative patients, surgeons must be aware of the possibility of late complications following multi-level posterior spinal fusion and should promptly address any changes in the patient’s neurological status. The differential diagnosis should include investigation for infection (5), hardware erosion and/or fusion failure.
We report on two rare cases where the upper instrumentation migrated toward the canal at 2 and 6 years postoperatively, causing spinal cord compression and progressive neurological deficit.
Case presentation
Clinical case 1
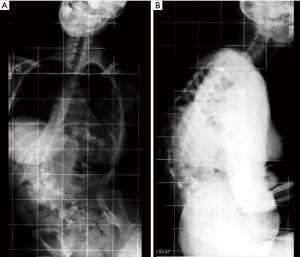
A 16-year-old female with Down syndrome underwent posterior spinal fusion for a 44º right thoracic curve (Figure 1). A T4–L4 posterior instrumentation was performed with no technical difficulties. A hybrid construct with a Universal Spinal System (USS) lateral-loading device was selected (Figure 2). Local and iliac bone graft was used. Proper placement of the implants was checked by post-operative radiographs. There were no surgical complications; somatosensory evoked potential (SSEP) was normal throughout the entire procedure, and the patient awoke without any neurological deficits.
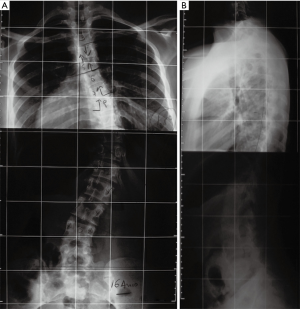
Two years after the index surgery and 3 months after a car accident, the patient started to have progressive difficulty in walking, exhibiting bilateral lower-extremity weakness, increased spasticity more severe on the left side, and poor balance. Her physical examination showed a slow and spastic gait with a wide base. There were no other symptoms such as fever or back pain. Long films were interpreted as normal with no significant changes from previous ones. Due to the context of Down syndrome, dynamic cervical spine films were ordered which showed no signs of instability. The white blood cell (WBC) count, C-reactive protein (CRP) and sedimentation rate (SR) were normal. Because it was impossible to perform magnetic resonance imaging, a CT scan was obtained when the patient became unable to walk, with global motor strength of 2/5 in the left lower extremity and 3/5 on the right. Clonus was evident and a Babinski’s was present on the left but equivocal on the right. The CT scan showed exuberant medial migration of the two upper-left proximal pedicle hooks into the canal with significant spinal cord compression (Figure 3A,B,C,D). No peri-implant fluid collections were observed. The patient was admitted and the instrumentation was promptly removed.
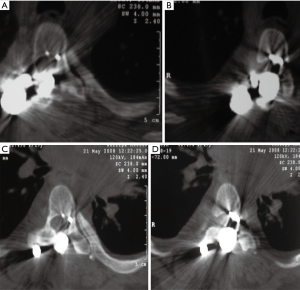
During surgery we realized that both pedicle hooks had migrated into the canal and were covered by a layer of bone, suggesting progressive bony remodeling during migration. There was no sign of acute fracture or pseudarthrosis. The patient had an uneventful recovery with early improvement of her neurological deficits. A post-operative spine MRI showed clear signs of cord edema at the level of T5. Cultures returned negative and at 6-month post-operative follow-up, the patient was able to walk without support having normal bowel and bladder function. No further procedure or surgery was undertaken.
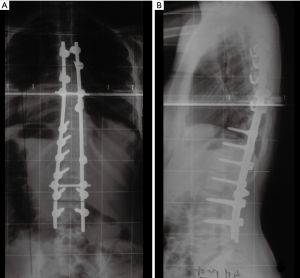
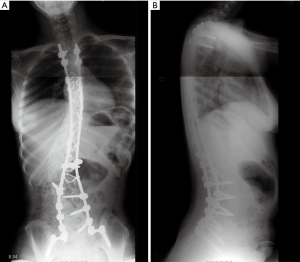
The patient is now eight years out from her last surgery with full neurological recovery and a stable residual deformity (Figure 4A,B).
Clinical case 2
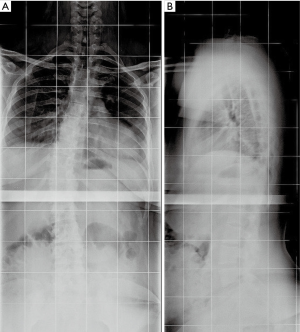
We were asked to re-evaluate a 19-year-old female with cerebral palsy (West syndrome). She was six years status-post uneventful T2-to-pelvis fusion for a 100º neuromuscular scoliosis (Figures 5,6). During the index surgery, we had used four screws proximally without cross-links, followed by sublaminar wires through the dorsal spine and screws in the lumbosacral area. Local bone graft was used and correct implant placement was confirmed by post-operative radiographs.
Three months before the requested clinical re-evaluation, the patient started complaining of discomfort over the proximal end of the instrumentation. Other than progressive weight loss there had been no other symptoms. One month before the consultation, the mother noticed a progressive decline in lower limb movement and some changes in the patient’s bladder habits (with a history of urinary tract infection). The mother denied any trauma or episodes of grand mal seizures in the patient’s history. She had experienced no fevers or other signs of current infection; the surgical scar was well healed. On physical examination, the patient was significantly under-nourished, was constantly drawing her hand toward her neck, and had no spontaneous movement of either lower extremity (though movement had been present before). Both lower extremities exhibited hypertonia and clonus, although it was difficult to compare this with previous observations.
On long spinal films, loosening around the two left-upper screws was evident (Figure 7A). A CT scan showed medial migration of these upper screws, which were grossly present within the spinal canal compressing the cord (Figure 7B,C). The WBC count was 9.87×109/L and CRP was 0.36 mg/dL. The patient was scheduled for implant removal, which was planned to be a small procedure removing only the dislocated implants.
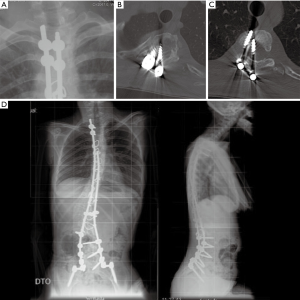
Corrosion debris was found at the time of surgery with a small amount of fluid around the stainless-steel implants. The rod was cut and proximal two screws were removed from the canal. The fluid was sent for culture and no further surgery was performed. The right side was left untouched as there was no loosening around those implants. Early cultures were negative, but at our request these were kept in culture for two additional weeks resulting in growth of Propionibacterium acnes. Appropriate antibiotics were given intravenously for over two weeks and were then continued orally for 3 months. At 2 years follow-up patient didn’t regain her previous lower extremity motion there was no infection recurrence and deformity remained stable (Figure 7D).
Discussion
These two unusual cases clearly show the need for long-term follow-up of patients who have had posterior spinal fusion for scoliosis, since neurological complications, although rare, can reportedly occur up to 15 years after the index procedure (1-4). The few cases reported in the English-language literature are mostly related to persistence of non-union with progression of deformity, a possible stretching effect on the spinal cord, or bony overgrowth into the vertebral canal at the level of the nonunion (1-3). In 2003, Tribus and Garvey et al., reported on a case of delayed infection 10 years after scoliosis surgery. During implant removal, total laminar erosion on the convex side was described with the rod in direct contact with the spinal cord. According to the authors, mechanical factors due to persistence of micro-motion were most likely eroding bone, which allowed implant intrusion into the canal (6). Hales et al. previously showed a problem with L5 distal-hook fixation in the Harrington era, due to remaining lumbosacral lordosis and persistent motion. A significant number of patients with neurological problems have experienced clinical resolution following implant removal (7). Vialle et al. also reported paralysis which developed ten years after the index procedure, caused by an unlikely leiomyosarcoma involving a laminar hook. He stated that any neurological deficit should be promptly investigated because of possible unusual implant-related complications (8).
The two remarkable cases we report herein represent atypical proximal instrumentation failure but with two distinctly different types of anchors. It is well recognized that a hybrid construct (hooks proximally/screws distally) is less able to correct and maintain a coronal deformity due to more frequent implant-related complications (9). Luo et al., in their systematic review compared pedicle-screw constructs with hybrid constructs, documented a significant increase in implant dislodgment with hybrid constructs, requiring operative correction. Only one case out of 663 patients sustained a nerve root injury (10). In addition, it has been our experience with hybrid constructs to observe some shift of the spine over the years, with some progression or compensation in the upper curve. This may be related to the increased difficulty in grafting this area due to the presence of hooks and cross links.
Our first patient (case 1 above) also had an increased risk for complications with instrumentation due to Down syndrome. Although there are very few case series describing scoliosis in this context, those cases have raised issues of infection, pseudarthrosis, and junctional problems. Milbrandt et al. documented complications in four out of seven patients (57%) with Down syndrome, who were treated surgically for scoliosis. These complications included three pseudarthroses, four implant failures, three cases of superior kyphosis, and one infection. Of the four implant failures, one was at a pseudarthrosis level, well seen on radiographs; the other three were at the superior or inferior limits of the constructs, with loss of instrumentation fixation (11). In the series by Lerman et al., also in seven patients, one pseudarthrosis was reported with hardware failure, as well as one distal lateral junctional subluxation, one delayed wound healing, one surgical-site infection and one upper hook pull-out (12). A more recent series by Abousamra et al. reported only one early deep infection in 10 patients with Down syndrome, who were operated on for scoliosis (13). Although there was a mean follow-up of 2.6 years, the authors addressed the possible relationship between a more stable construct with screws and a better outcome; most of the previous cases reported in the literature used hybrid constructs, Harrington rods, or even anterior Dwyer types of instrumentation (11,12).
The authors are most familiar with hook failure via pulling out or by fracturing the laminae, but medial migration into the spinal canal, as we report, is a rare and less-expected complication. Although a recent trauma (the auto accident) may have contributed to the development of neurological compromise, we believe most of the observed migration most likely happened over a period of time since the implants were well settled, perfectly covered by bone, and not loose.
In our second case, looking at sequential long spinal films during follow-up, we did realize the presence of rotation along the dorsal spine most likely due to a crankshaft phenomenon, since a CT scan showed a regular fusion mass at the apex of the dorsal curve. Due to this rotation, upper screws may have been put in to mechanical stress which, combined with typical poor bone mineral density in this patient, may have contributed to screw loosening and failure followed by cavitation. On the other hand, this case resembles others reported by Richman et al. and Beguiristain et al., since we found metal debris, a small amount of corrosion, along with the presence of Propionibacterium acnes, all of which could have helped cause bone resorption and fixation failure (14,15).
In summary, late paralysis following posterior spinal fusion—although rare—should lead the attending physician to fully evaluate the spine for diagnoses such as non-union, infection and/or possible implant migration into the spinal canal. It is impractical to follow all neuromuscular surgical patients for many years after routine follow-up care has been completed. However, both patients and their families should be educated about the remote possibility of this type of late complication so they may seek medical care if there are changes in the patient’s baseline neurological function.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Each patient’s mother was informed that data concerning their daughter’s cases would be submitted for publication; both agreed to this.
References
- Eismont FJ, Simeone FA. Bone overgrowth (hypertrophy) as a cause of late paraparesis after scoliosis fusion. A case report. J Bone Joint Surg Am 1981;63:1016-9. [Crossref] [PubMed]
- Court-Brown CM, McMaster MJ. Pseudarthrosis: a late cause of paraparesis after scoliosis surgery. A case report. J Bone Joint Surg Am 1982;64:1246-8. [Crossref] [PubMed]
- Savini R, Di Silvestre M, Gargiulo G. Late paraparesis due to pseudarthrosis after posterior spinal fusion. J Spinal Disord 1990;3:427-32. [PubMed]
- Roy DR, Huntington CF, MacEwen GD. Pseudarthrosis resulting in complete paraplegia fifteen years after spinal fusion. Arch Orthop Trauma Surg 1984;102:213-5. [Crossref] [PubMed]
- Choma T, Burke M, Kim C, et al. Epidural abscess as a delayed complication of spinal instrumentation in scoliosis surgery: a case of progressive neurologic dysfunction with complete recovery. Spine (Phila Pa 1976) 2008;33:E76-80. [Crossref] [PubMed]
- Tribus CB, Garvey KE. Full-thickness thoracic laminar erosion after posterior spinal fusion associated with late-presenting infection. Spine (Phila Pa 1976) 2003;28:E194-7. [Crossref] [PubMed]
- Hales DD, Dawson EG, Delamarter R. Late neurological complications of Harrington-rod instrumentation. J Bone Joint Surg Am 1989;71:1053-7. [Crossref] [PubMed]
- Vialle R, Wolff S, David P, et al. Late paraplegia after scoliosis treatment: an uncommon diagnosis. J Spinal Disord Tech 2005;18:531-4. [Crossref] [PubMed]
- Kim YJ, Lenke LG, Kim J, et al. Comparative analysis of pedicle screw versus hybrid instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31:291-8. [Crossref] [PubMed]
- Luo M, Li N, Shen M, et al. Pedicle screw versus hybrid instrumentation in adolescent idiopathic scoliosis: A systematic review and meta-analysis with emphasis on complications and reoperations. Medicine (Baltimore) 2017;96:e7337. [Crossref] [PubMed]
- Milbrandt TA, Johnston CE 2nd. Down syndrome and scoliosis: a review of a 50-year experience at one institution. Spine (Phila Pa 1976) 2005;30:2051-5. [Crossref] [PubMed]
- Lerman JA, Emans JB, Hall JE, et al. Spinal arthrodesis for scoliosis in Down syndrome. J Pediatr Orthop 2003;23:159-61. [Crossref] [PubMed]
- Abousamra O, Duque Orozco MDP, Er MS, et al. Scoliosis in Down's syndrome. J Pediatr Orthop B 2017;26:383-7. [Crossref] [PubMed]
- Beguiristain J, del Río J, Duart J, et al. Corrosion and late infection causing delayed paraparesis after spinal instrumentation. J Pediatr Orthop B 2006;15:320-3. [Crossref] [PubMed]
- Richman SH, Razzano AJ, Morscher MA, et al. Metallosis Presenting as a Progressive Neurologic Deficit Four Years After a Posterior Spinal Fusion for Adolescent Idiopathic Scoliosis: A Case Report. Spine (Phila Pa 1976) 2017;42:E56-9. [Crossref] [PubMed]
Cite this article as: Fernandes P, do Brito JS, Monteiro J. Late implant migration with neurologic compromise as a complication of scoliosis surgery. AME Case Rep 2019;3:1.


