Papillary thyroid carcinoma with bilateral axillary lymph node involvement: a case report outlining hypothesis for locoregional spread and therapeutic implications
Introduction
Papillary thyroid carcinoma (PTC) commonly spreads through the lymphatics to nearby cervical lymph nodes (1). Distant metastatic spread is rare (2–3%) and spread to the axillary lymph nodes is even more rare, scarcely described in the literature. Locoregional spread from PTC to the axillae can be associated with cervical bulky lymph node disease and/or skin involvement, altering lymphatic drainage. We propose these pathways demonstrate axillary lymph node involvement (ALNI) in PTC to be locoregional disease, rather than distant metastasis, the management of which may have significant therapeutic implications. We report a case of classic type PTC with extensive cervical lymph node involvement, local extension to the skin of the neck and bilateral ALNI without evidence of distant disease.
Case presentation
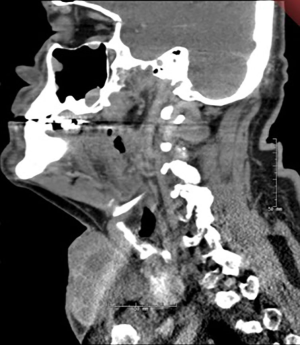
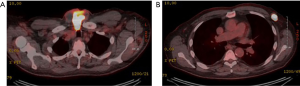
The patient is a 71-year-old man with no known family history of endocrinopathies or thyroid carcinoma, and no exposure to radiation, who initially presented with an enlarging midline neck mass found to be intimately associated with the skin. A CT of the neck showed a 5 cm × 8.5 cm × 7 cm heterogeneous mass in the low anterior neck inseparable from the enlarged multinodular thyroid gland in addition to bilateral level IV, left level II, and left intraparotid lymphadenopathy (Figure 1). Fine needle aspiration (FNA) of the mass was consistent with PTC, classic type. A PET/CT showed a PET-avid anterior midline neck mass, involved skin, with associated bilateral cervical lymphadenopathy in addition to bilateral axillary adenopathy (Figure 2A,B). On physical exam, the patient had a palpable level I 2.5 cm mass in the left axilla and no distinct palpable masses in the right axilla to correlate with PET/CT scan findings. FNA of the left axilla mass was consistent with thyroid carcinoma. The patient underwent a total thyroidectomy, central and bilateral modified radical neck dissection, and, separately, bilateral axillary dissection. Final pathology was consistent with classic PTC with lymphatic invasion and 28 of 82 lymph nodes positive for PTC and extra-nodal extension (pT4aN1b). Axillary pathology showed metastatic PTC in 3 positive lymph nodes on the right and 5 positive lymph nodes on the left. After surgical resection, the patient began radioactive iodine (RAI) therapy. He remains disease free 1 year later.
Discussion
PTC is the most common type of well differentiated thyroid carcinoma, and has a propensity for lymphatic metastasis, but extremely rare distant hematogenous spread. Regional lymph nodes at risk are central neck (level VI), lateral neck (usually levels II–V), and top mediastinal (level VII). Clinically relevant cervical lymph node macrometastases is estimated to occur in 20–50% of patients (1-4) while less significant micrometastases (2 mm) occur in up to 90% of patients with PTC (3,5,6). Meticulous surgical resection of all macroscopic disease remains the mainstay of treatment for PTC. By American Thyroid Association 2015 guidelines, near-total or total thyroidectomy in addition to resection of all gross cervical nodal metastatic disease is recommended to both clear the tumor burden and optimize the use of adjuvant therapies such as RAI or novel systemic therapies (6).
However, management and surgical strategy becomes challenging when lymph node basins outside of levels I–VII are involved. Axillary lymph node metastasis is considered distant lymph node involvement, implies hematogenous spread, and thus it is stage 4 disease (6). Most cases reported in the literature present ALNI in the setting of recurrent disease and accompanying other foci of distant metastatic disease. In this setting, ALNI naturally is considered part of the distant disease process and stage 4 disease (7-10). Our patient’s clinical presentation is that of ALNI synchronous with his primary disease, with two characteristics that may offer an anatomical explanation and paradigm of ALNI as extension of loco-regional disease as opposed to hematogenous spread.
The first clinically important feature is skin involvement. Once skin is involved, lymphatic drainage of tumor in the skin follows patterns similar to melanoma. Lymphatic spread from head and neck melanoma is remarkably variable and robust, with one report estimating multiple lymph node basins involved in 66.7% of patients (11). Patterns of lymphatic drainage can be complex and variable (12). In a study of such drainage patterns utilizing lymphoscintigraphy, Fitzgerald et al. defined a subset of five patients with head and neck melanoma and bilateral axillary sentinel nodes, demonstrating that both nodal basins are locally accessible (11).
Such complex relationships of lymph node drainage from axial tumors have been better elucidated in the breast and head and neck cancer literature. Kiluk et al. demonstrated in patients with contralateral axillary nodal involvement, 77% of patients had dermal involvement of the breast primary (13). Lymphoscintigraphy demonstrates lymphatic drainage outside the axilla in about 20–30% of breast cancer patients, particularly when the radiolabel is injected intraparenchymally (14). Lymphatics from skin of the chest wall may indeed be visualized crossing the midline after injection of isosulfan blue dye or lymphoscintigraphy as well, illustrating the anatomic connections to the contralateral chest (15). This concept has further been demonstrated in head and neck squamous cell carcinomas (SCC). In fact, Kowalski et al. identified that axillary metastases were found at autopsy in 2–9% of patients who died of head and neck SCC, and these findings were frequently in association with skin implantation by the primary SCC (16).
The second important clinical feature of our case is the extensive burden of disease in the cervical lymph nodes, potentially altering lymphatic drainage. Lymphatic drainage can be altered by malignant obstruction due to bulky disease, and/or by previous surgery, scarring or radiation. It has been proposed that when nodes in the jugulo-subclavian confluence are involved with carcinoma and lymphatic flow is obstructed, disease spreads retrograde along the transverse cervical lymph nodes in the supraclavicular region (17). These retrograde pathways of lymphatic drainage can culminate in axillary lymph node metastasis. In breast cancer, similar to PTC, contralateral axillary lymphadenopathy is often associated with patients that have undergone a previous lymph node dissection, had radiation or had extensive bulky disease (18-20). Similarly in cases of SCC of the head and neck with axillary metastases, risks of axillary metastases include extensive burden of disease in the neck, as well as previous surgery and radiation (16,21).
In light of the aforementioned literature, anomalous spread of cancer to the axilla from axial tumors (head, neck and trunk) may represent limited locoregional disease or may occur in the setting of distant metastatic disease. This notion has been difficult to study in any cancer setting given the rarity of the presentation. With regards to PTC, lymphatic spread to the axilla is exceptionally scarce and only a handful of case reports in the literature exist (7-10,17,22,23). However, amongst these reports exist patients with very long disease free intervals prior to their axillary recurrences and a small subset without additional organ involvement (17) as in our patient. Such patients suggest that there may be a window of local extension to axillary lymph nodes prior to systemic involvement, whereby complete surgical resection represents a curative approach. Furthermore, taking into account the biology of classic type PTC, hematogenous spread is extremely unlikely. Immediate assumption of ALNI as stage IV can result in undertreatment. However, recognizing that ALNI can be a reflection of anatomic changes in lymphatic drainage due to skin involvement and bulky lymphadenopathy rather than hematogenous spread can explain ALNI in an otherwise biologically indolent cancer. Our case and literature review highlights risk factors for axillary metastasis which include skin involvement, bulky cervical lymph node disease, previous surgery and radiation. In these high-risk patients, appropriate staging should include a PET/CT scan. We recognize the same patients who are at risk for axillary metastasis are also at higher risk for distant metastatic disease and thus must be correctly evaluated. In the absence of distant metastatic disease, a standard axillary dissection should be performed to render the patient disease-free with curative intent, and may provide a survival advantage. In the setting of distant metastatic PTC, completeness of surgical resection of all accessible loco-regional disease remains exceptionally important to facilitate effectiveness of RAI treatment. Hence, even in the metastatic setting, a standard axillary dissection should be performed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 1996;5:43-63. [Crossref] [PubMed]
- Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid? Prognostic significance of lymph node metastasis and multifocality. Cancer 2003;98:31-40. [Crossref] [PubMed]
- Arturi F, Russo D, Giuffrida D, et al. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol Metab 1997;82:1638-41. [Crossref] [PubMed]
- Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg 1994;18:559-67; discussion 567-8. [Crossref] [PubMed]
- Qubain SW, Nakano S, Baba M, et al. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery 2002;131:249-56. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Krishnamurthy A, Vaidhyanathan A. Axillary lymph node metastasis in papillary thyroid carcinoma: report of a case and review of the literature. J Cancer Res Ther 2011;7:220-2. [Crossref] [PubMed]
- Koike K, Fujii T, Yanaga H, et al. Axillary lymph node recurrence of papillary thyroid microcarcinoma: report of a case. Surg Today 2004;34:440-3. [Crossref] [PubMed]
- Angeles-Angeles A, Chable-montero F, Martinez-benitez B, et al. Unusual metastases of papillary thyroid carcinoma : report of 2 cases. Ann Diagn Pathol 2009;13:189-96. [Crossref] [PubMed]
- Ers V, Galant C, Malaise J, et al. Axillary lymph node metastasis in recurrence of papillary thyroid carcinoma: A case report. Wien Klin Wochenschr 2006;118:124-7. [Crossref] [PubMed]
- Fitzgerald TL, Gronet EM, Atluri P, et al. Patterns of node mapping differ for axial and extremity primary cutaneous melanoma: A case for a more selective use of pre-operative imaging. Surgeon 2016;14:190-5. [Crossref] [PubMed]
- Uren RF, Thompson JF, Howman-giles R. The Role of Lymphoscintigraphy in the Detection of Lymph Node Drainage in Melanoma. Surg Oncol Clin N Am 2006;15:285-300. [Crossref] [PubMed]
- Kiluk JV, Prowler V, Lee MC, et al. Contralateral axillary nodal involvement from invasive breast cancer. Breast 2014;23:291-4. [Crossref] [PubMed]
- Tanis PJ, Nieweg OE, Valde RA. Impact of non-axillary sentinel node biopsy on staging and treatment of breast cancer patients. Br J Cancer 2002;87:705-10. [Crossref] [PubMed]
- Huston TL, Pressman PI, Moore A, et al. The presentation of contralateral axillary lymph node metastases from breast carcinoma: a clinical management dilemma. Breast J 2007;13:158-64. [Crossref] [PubMed]
- Kowalski LP. Noncervical lymph node metastasis from head and neck cancer. ORL J Otorhinolaryngol Relat Spec 2001;63:252-5. [Crossref] [PubMed]
- Nakayama H, Wada N, Masudo Y, et al. Axillary Lymph Node Metastasis from Papillary Thyroid Carcinoma: Report of a Case. Surg Today 2007;37:311-5. [Crossref] [PubMed]
- van der Ploeg IM, Oldenburg HS, Rutgers EJ, et al. Lymphatic Drainage Patterns from the Treated Breast. Ann Surg Oncol 2010;17:1069-75. [Crossref] [PubMed]
- Boughey JC, Ross MI, Babiera GV, et al. Sentinel Lymph Node Surgery in Locally Recurrent Breast Cancer. Clin Breast Cancer 2006;7:248-53. [Crossref] [PubMed]
- Barranger E, Talbot JN, Uzan S. CASE REPORT Contralateral Axillary Sentinel Lymph Node Drainage in Breast Cancer: A Case Report. J Surg Oncol 2004;86:167-9. [Crossref] [PubMed]
- Oo AL, Yamaguchi S, Iwaki H, et al. Axillary nodal metastasis from oral and maxillofacial cancers: a report of 3 cases. J Oral Maxillofac Surg 2004;62:1019-24. [Crossref] [PubMed]
- Kamaleshwaran KK, Rajan F, Mohanan V, et al. Rare case of axillary lymph node metastasis in papillary thyroid carcinoma detected using Iodine-131 whole-body scintigraphy and single-photon emission computed tomography/computed tomography. Indian J Nucl Med 2015;30:168-70. [Crossref] [PubMed]
- Kepenekci I, Demirkan A, Cakmak A, et al. Axillary lymph node metastasis as a late manifestation of papillary thyroid carcinoma. Thyroid 2009;19:417-9. [Crossref] [PubMed]
Cite this article as: Caso R, Villano AM, Sutton W, Alexander R, Caragacianu DL. Papillary thyroid carcinoma with bilateral axillary lymph node involvement: a case report outlining hypothesis for locoregional spread and therapeutic implications. AME Case Rep 2019;3:2.