
Discovery of thoracic osteoma after elective lumbar spinal surgery: a case report
Introduction
Osteomas are described as dense, benign, well-differentiated tumors of mature lamellar bone usually arising from a cortical surface. Osteomas can be divided into “ivory” when made from uniformly dense bone or “cancellous” when primary composition is of spongy bone (1). The most common reported location is within the skull or paranasal sinuses, where the prevalence is believed to be approximately 0.42% (2). In patients receiving biopsies of primary bone tumors, the prevalence is estimated to be approximately 0.03% (3). This statistic, however, is difficult to measure because of the asymptomatic nature of these tumors and the fact that most are discovered incidentally on routine imaging. If symptoms occur, these may be from cosmesis or compression of a nearby structure such as sinusitis (3).
Osteomas are usually solitary lesions. In cases of multiple osteomas, a diagnosis of Gardner syndrome may be considered. On radiographic imaging, osteomas will appear as homogenous radio-opaque round or ovoid lesions with smooth cortical borders and are typically smaller than 3 cm in diameter. They will often emerge from the outer surface of bone and do not demonstrate surrounding periosteal reaction based on their benign nature. Histologically, they demonstrate dense lamellar or cortical bone (3,4).
Primary benign tumors of the spine are exceedingly rare and comprise approximately 1% of all primary skeletal tumors (5). This 1% is comprised of lesions including osteoid osteoma, eosinophilic granuloma, aneurysmal bone cysts, osteoblastoma, osteochondroma, and giant cell tumors of bone (6). A case report from 1998 reported the first case of osteoma within the vertebral column at the C6 pedicle (1). A case series from 2006 only found seven cases within the English literature of primary intra-canal osteomas and discussed two more causing post-traumatic symptoms in the cervical spinal canal (2). The largest available case series was published in 1996 and included 11 cases of extra-cranial osteoma, only five of which occurred in the spine. Of these five, there were no cases of thoracic osteoma (one cervical, two lumbar, and two sacral) (3). On literature review, there were no articles found within the last decade regarding spinal osteomas.
Here, the authors present the first case of thoracic osteoma discovered subsequent to a lumbar laminectomy/discectomy for symptomatic lumbar radiculopathy.
Case presentation
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
The patient is a 47-year-old otherwise healthy male. He is gainfully employed in a warehouse moving boxes. The onset of lower-extremity radiculopathy symptoms was gradual approximately 4–5 months prior to presentation to the emergency department. He reported no history of trauma. The right leg felt more symptomatic than the left, with his complaints being of “electric shock” sensations radiating down the posterior leg down his calf. The pain was exacerbated by motion or a long day at work, and only partially relieved by rest. The patient denied any symptoms of myelopathy or cauda equina syndrome, including bowel or bladder incontinence. On further questioning, he did endorse recent sexual dysfunction.
On physical exam, the patient’s upper extremities were neurovascularly intact without abnormal reflexes. His lower extremity exam was pertinent for weakness in the right side entirely. Iliopsoas and quadriceps were graded 2/5 motor strength. Tibialis anterior, extensor hallucis, and gastrocnemius/soleus complex were all 3/5. He had intact sensation to light touch bilaterally from L2-S3. His rectal tone was intact as was peri-anal sensation. His patellar reflexes were brisk and symmetric at 3+.
At the time of presentation, a lumbar spine MRI was obtained by the emergency department with findings of large L3-4, L4-5, and L5-S1 herniated nucleus pulposus as well as spinal stenosis with compression of the cauda equina (Figure 1). The patient was admitted and taken for a L3-S1 laminectomy, discectomy, and posterior spinal fusion as well as L3-4/L5-S1 transforaminal interbody fusion. The patient tolerated the procedure well and was discharged from the hospital on postoperative day 8 with recovery of strength to grades 3–4 in all myotomes in the RLE. His radiculopathy had resolved.
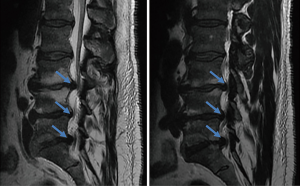
On postoperative day 16 the patient returned to the emergency department with the same symptoms that he had preoperatively. He was seen by another physician and MRI’s of the thoracic and lumbar spine were ordered. The lumbar spine imaging showed only postoperative changes with a seroma posterior to the fusion. No further disk herniations or cord compression was identified at the lumbar levels. The thoracic spine imaging demonstrated a dense, cortical lesion posterior to the thecal sac but anterior to the laminae at the T3 level. There was no surrounding edema, and its signal quality matched that of bone on both T1 and T2 weighted images. It spanned approximately 1.2×1 cm at its largest dimensions and was contained entirely within the spinal canal (Figure 2). This MRI was followed with a thoracic spine CT scan confirming the dense, osseous nature of the mass and suggested the possibility of osteoma arising from the facet joints at T3-4.
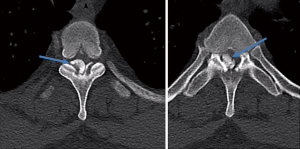
The patient was taken the next day for thoracic spine decompressive laminectomy with three-level posterior spinal fusion from T2-T4. The mass in its entirety was submitted for pathologic analysis. Postoperatively, the patient had immediate symptom relief. His subjective radiculopathy improved, and his motor strength was 3-4/5 in the right lower extremity. He was discharged from the hospital without incident on postoperative day 4. At 2-month follow-up, the patient has regained his strength to 4/5 throughout the right lower extremity and is about to return to work on “light-duty”. At 6-month follow-up, he has returned to work. He remains 4+/5 strength in the right lower extremity. He has no subjective numbness. He does not use any assistive device for ambulation. He is not taking any pain medication.
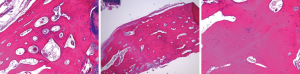
The histopathology of the lesion was reviewed by the hospital’s pathology department. The sample demonstrated mature, lamellar bone consistent with osteoma (Figure 3). No nidus was found to suggest an osteoid osteoma. No malignant-appearing cells were present to suggest parosteal osteosarcoma. No new-bone formation was found to suggest a post-traumatic etiology. The diagnosis of osteoma was confirmed by the pathologic sample.
Discussion
Presented here is a case of discovering a thoracic spine lesion following lumbar spinal surgery. In summary, the presented to the hospital with long-standing symptoms of lumbar stenosis with superimposed disc disease. Preoperatively he was experiencing neurologic symptoms including weakness and radiculopathy. He did not treat these symptoms with medication preoperatively. Due to the severe nature of his symptoms, he was indicated for spinal a decompression and fusion. While the patient recovered from his lumbar spine surgery, he encountered a “second-hit” in which his chronic thoracic spine lesion became symptomatic causing neurologic weakness of his lower extremities. Appropriate imaging was obtained leading to a preliminary diagnosis of a mass within the thoracic spinal canal. This mass was excised en-bloc and submitted to pathology for diagnosis, which confirmed a benign osteoma.
The patient’s lesion would likely not have been discovered had his lumbar stenosis and herniated disks not become symptomatic. It is the authors’ belief that the decompression of the cauda equina at caudal levels caused a more even distribution of cerebrospinal fluid (CSF) throughout the cord and subsequent expansion. With this alteration in fluid mechanics, the compression at the level of the pre-existing osteoma immediately became more severe and led to symptom progression.
Wang et al. discussed two cervical spine osteomas that were not symptomatic until those patients experienced significant trauma (i.e., motor vehicle accidents). Therefore, the trauma itself may have caused the symptoms to evolve more quickly (2). They state that the aim of surgical management of osteomas includes decompression and subsequent stabilization if instability exists. In our case, access to the posterior osteoma required a full laminectomy and facetectomy and necessitated a three-level thoracic spine fusion.
In Peyser’s case series of 11 osteomas, less than half were found in the spinal canal. Overall, the only patient who did not experience symptomatic relief after resection was the patient with a sacral osteoma, most likely secondary to incomplete resection with subsequent regrowth. They propose successful treatment with excisional biopsy and marginal resection of the tumor (3). A benefit of this series is the fact that they had long-term follow-up and demonstrated no recurrence of the osteoma in their patients despite marginal resection. Histologically, intra-canal osteomas did not differ from those encountered in the skull. They demonstrated the same benign nature and histologic characteristics as the osteomas found elsewhere.
In our case of thoracic osteoma, it is unlikely that the patient’s symptoms will recur. Even if sub-total resection was completed, the slow-growing nature of the tumor will likely not cause repeat cord-compression. In a multicenter cohort study of 84 patients treated surgically over 31 years, recurrence of symptoms occurred in only 6% of patients (7). Furthermore, the patient’s symptoms were likely a two-hit-phenomenon where his severe lumbar disease was causing compression and nerve root symptoms in tandem with the thoracic compression. At the submission of this article, approximately 6 months postoperatively, the patient has had full resolution of his symptoms. Without the lumbar spinal surgery, it is likely that he would have presented much later in the disease process and possibly suffered irreversible spinal cord injury. This theory is supported by the history of trauma in nearly all case reports of spinal osteoma—a first hit may be required to incite symptomatology.
Conclusions
Osteomas are slow-growing, benign tumors rarely found in the spinal canal. Infrequently, these lesions cause symptoms secondary to their abutting nearby structures. Recent spinal surgery at another level may alter cerebrospinal/intrathecal pressure enough to cause new-onset compression at a more proximal level and exacerbate neurologic symptoms from these indolent growths. Decompression with a total or subtotal excision is a successful treatment option for intra-canal thoracic osteoma.
Acknowledgements
We acknowledge Dr. Parisa Javidian for her histologic analysis and digital photographs. Her time and expertise are greatly appreciated.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
References
- Rengachary SS, Sanan A. Ivory osteoma of the cervical spine: case report. Neurosurgery 1998;42:182-5. [Crossref] [PubMed]
- Wang W, Kong L, Dong R, et al. Osteoma in the upper cervical spine with spinal cord compression. Eur Spine J 2006;15:616-20. [Crossref] [PubMed]
- Peyser AB, Makley JT, Callewart CC, et al. Osteoma of the long bones and the spine. A study of eleven patients and a review of the literature. J Bone Joint Surg Am 1996;78:1172-80. [Crossref] [PubMed]
- White LM, Kandel R. Osteoid-producing tumors of bone. Semin Musculoskelet Radiol 2000;4:25-43. [Crossref] [PubMed]
- Thakus NA. GLUT-1. J Am Acad Orthop Surg 2013;21:65-6. [PubMed]
- Bahloul K, Xhumari A, Feydy A, et al. Thoracic spine osteoid osteoma. Eur J Radiol 2003;46:74-7.
- Quraishi NA, Boriani S, Sabou S, et al. A multicenter cohort study of spinal osteoid osteomas: results of surgical treatment and analysis of local recurrence. Spine J 2017;17:401-8. [Crossref] [PubMed]
Cite this article as: Forlizzi J, Mennona S, McDonnell M, Chiappetta G, Rogalski S. Discovery of thoracic osteoma after elective lumbar spinal surgery: a case report. AME Case Rep 2019;3:10.


