
Case report of a neuroendocrine tumor of the thyroid gland with limited calcitonin expression: a diagnostic challenge
Introduction
Medullary thyroid carcinoma (MTC) is the most common neuroendocrine tumor of the thyroid gland and accounts for 1–2% of all thyroid cancers in United States according to the National Cancer Institute’s SEER data (1). MTCs originate from parafollicular cells or C-cells, which usually produce calcitonin. Consequently, calcitonin positivity is the most important criterion for diagnosis of MTC clinically and pathologically, and is also important for detection of disease recurrence after treatment.
Diagnosis of MTC when calcitonin staining is focal or absent can be challenging. It has even been suggested that it is impossible to define a tumor as MTC if it does not produce calcitonin (2). Tumors without calcitonin positivity may represent different cells of origin and differences in disease prognosis and recurrence (3,4). Such “atypical MTC” is rare. Consequently, the prognosis and definitive guidelines for management are lacking.
We present a case of a neuroendocrine tumor in the thyroid with only focal calcitonin staining on immunohistochemistry. The assumption of C-cell origin was made from positive staining for carcinoembryonic agent (CEA), thyroid transcription factor 1 (TTF-1) and paired box 8 (PAX8) without expression of thyroglobulin (Tg). We also review the literature of similar cases and discuss possible management approaches.
Case report
A 60-year-old woman presented with a 4 cm left thyroid nodule, which was found incidentally on MRI. Her past medical history was notable for history of breast cancer, right mastectomy with sentinel lymph node biopsy followed by a course of adjuvant chemotherapy, and one year of trastuzumab therapy. She continues to receive anti-hormonal treatment.
Her left thyroid nodule was found incidentally on her surveillance MRI for breast cancer. She was asymptomatic, but on physical examination, had a palpable left thyroid nodule that moved with deglutition. No cervical lymphadenopathy was detected. Her thyroid function tests were within normal limits. Neck ultrasound showed a dominant, hypoechoic, solid, hypervascular 2.9 cm left thyroid nodule without any suspicious cervical lymph nodes. According to the 2015 ATA guidelines, these ultrasound features are considered intermediate suspicion; hence, fine needle aspiration (FNA) was recommended for this dominant nodule. Additional small right thyroid solid nodules measuring up to 8 mm without suspicious features were also detected.
The initial FNA was inconclusive as there were only lymphoid tangles with no follicular epithelium. FNA was repeated a month later and showed clusters of small epithelial cells in a microfollicular pattern, with a large number of cells with spindle cell morphology. The differential diagnosis included medullary carcinoma and follicular neoplasm (Bethesda IV). Correlation with serum calcitonin level was recommended. Serum calcitonin was then measured using the Siemens (DPC) Chemilunescent method, which showed mild elevation (11 pg/mL; normal range ≤5 pg/mL).
After the risks and benefits of genetic testing were discussed with the patient, another FNA sample was submitted for AFIRMA Gene Expression Classifier (GEC) testing (Veracyte, Inc, South San Francisco, California) due to suspicion for MTC. The results were interpreted as suspicious but negative for MTC because a gene expression signature of MTC was not identified.
Four months later, another FNA was done on the same thyroid nodule for another round of gene testing. The FNA results indicated benign disease. Testing with the ThyGenX tm Oncogene Panel (Interpace Diagnostics, Inc, Parsippany, New Jersey) showed no detected mutation. Because of the worrisome feature, spindle cells morphology, reported in her previous FNA sample, serum calcitonin was again tested and found to be elevated (10–11 pg/mL). Because of the diagnosis uncertainty, although it was suspected, the patient decided to undergo a diagnostic left thyroid lobectomy. Intra-operatively, there was a large left thyroid nodule with no findings suggestive of local invasion or lymph node metastasis. There were no post-operative complications and she was discharged home the same day.
On pathology, a 3.8 cm × 3.1 cm × 2.7 cm, well-circumscribed, partially encapsulated nodule was identified. The tumor was composed of bland plump spindled to stellate cells with ovoid nuclei, irregular nuclear contours, fine vesicular chromatin and moderate pale to amphophilic cytoplasm, arranged in solid architectural pattern (Figure 1). The mitotic figures were inconspicuous (<1/10 high power fields) and there was no atypical mitotic figures or necrosis.
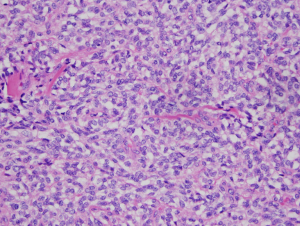
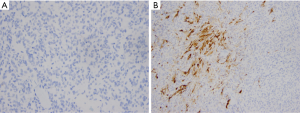
All margins were negative. The spindle cells stained positive for thyroid transcription factor 1 (TTF-1), PAX8, monoclonal and polyclonal CEA, synaptophysin and chromogranin (Figure 2). These cells were negative for thyroglobulin and focally positive for pan-cytokeratin and only focally positive for calcitonin (Figure 3). Ki67 showed a low proliferation of <1%. Overall, the morphology and immune profile supported the diagnosis of a neuroendocrine tumor (NET) of the thyroid gland with limited calcitonin expression.

The patient was referred for genetic consultation because her personal history of multiple sites of cancer (breast and thyroid) was suggestive of hereditary cancer predisposition. In addition, she had a maternal grandfather who was diagnosed with prostate cancer and a mother with a diagnosis of breast cancer. The patient’s blood sample was sent for sequencing, deletion, and duplication analysis of 130 genes, including BRCA1/2, KIT, MEN1, NF1, NF2, RET, SDHB and also TERT. The results were negative.
At the first follow-up visit, 1 month after surgery, the patient’s serum calcitonin level of 2 pg/mL was lower than the pre-operative value, of 10–11 pg/mL. After discussion by our institution’s tumor board, the decision was made to monitor her calcitonin and CEA levels. No further surgery for the other lobe was indicated given the non-aggressive features of the tumor as described in pathological findings and the somatic mutation testing result. Routine ultrasound of the cervical area was also suggested. At her latest follow-up visit, 8 months after surgery, her calcitonin level was 3 pg/mL and her CEA was 1.9 (Abbott Architect Chemiluminescent Microparticle Immunoassay, CMIA, ref range <5.1). Her last (7 months post-operatively) cervical ultrasound also showed no suspicious lymphadenopathy and no changes in previous small benign nodule in the right thyroid lobe.
Discussion
For pre-operative diagnosis of thyroid lesions, FNA is a useful and safe procedure, but diagnosis of MTC remains challenging. A previous meta-analysis found that the accuracy of FNA for diagnosing MTC in patients with a suspicious nodule was under 50%, partly because the tumor is rare and has diverse cytological characteristics (5). However, immunocytochemical staining (ICC) and calcitonin measurement in needle wash-out fluid from FNA have increased the accuracy of diagnosis (5,6). According to the meta-analysis, the sensitivity and specificity of calcitonin measurement in aspiration needle wash-out fluid and ICC for calcitonin are 96.3% and 92.3%, respectively (5). Another systematic review demonstrated that almost all MTC lesions can be correctly detected by measurement of calcitonin in wash-out fluid from FNA (7). Recent ATA guidelines state that, “FNA findings that are inconclusive or suggestive of MTC should have calcitonin measured in the FNA wash out fluid and IHC staining of the FNA sample to detect the presence of the marker such as Ctn, chromogranin and CEA and the absence of thyroglobulin.” (1). However, the issue remains of how to select patients who should have FNA calcitonin measured.
In our patient, molecular testing was considered instead of proceeding directly with surgery both to avoid an unnecessary operation on a benign nodule and to ensure adequate surgical treatment in case of malignancy because the predicted risk of malignancy in Bethesda IV is about 15–30% (8). Although the first round of molecular testing using Afirma GEC-s resulted in a suspicious for malignancy, further testing with Afirma MTC was negative. The Afirma GEC uses microarray technology to assess the mRNA expression profiles of indeterminate thyroid nodules. The test is known to have a high negative predictive value (NPV) that has been validated in a blinded multicenter prospective trial (9). However, it has a low positive predictive value (PPV). Altogether, benign GEC results have the most meaningful impact on management because for nodules with pretest probability of malignancy <25%, the high NPV of a benign GEC result reduces the risk of malignancy to <6%, which makes it safe for non-operative management. A patient with suspicious GEC results should still be considered for diagnostic lobectomy with the expectation that 60% of these nodules will be histologically benign (9). Afirma MTC analyzes the expression of 5 genes that are expressed in MTC: Calcitonin related polypeptide-alpha (CALCA), carcinoembryonic antigen related cell adhesion molecule 5 (CEACAM5), secretogranin III (SCG3), sodium channel voltage-gated type IX alpha subunit (SCN9A) and synaptotagmin IV (SYT4) (9). However, a negative Afirma MTC result does not significantly change the risk of malignancy of an Afirma GEC suspicious result.
The second round of molecular testing on our patient, using ThygenXTM Oncogene Panel Status, also showed no detectable mutations. The test uses multiplex PCR and detection of mutations (BRAF, HRAS, NRAS and KRAS) and rearrangements (RET-PTC1, RET-PTC3 and PAX8-PPARG) by sequence-specific probes (9). Although a negative result predicts benign pathology in 80% of cases, malignancy cannot be excluded (10) because other rare mutation beside genes listed in the panel may be present but not detected. In addition, because a thyroid nodule can contain multifocal areas of heterogeneous pathology, sampling variation may occasionally result in under diagnosis. This ultimately still leads to surgical excision.
Calcitonin is a sensitive and specific marker used for diagnosis and surveillance for recurrence of MTC because its serum concentration relates directly to C-cell mass. Calcitonin level is also predictive for tumor size (11). Lack of calcitonin expression both in serum and from tissue immunohistochemistry is a rare condition as reported in the series of 839 MTC patients, only 0.83% of patients were reported to have non-secretory MTC (12). This can make diagnosis and management difficult. In patients suspected to have MTC whose serum calcitonin level is either low or normal, MTC cannot be excluded (13-15).
Other studies have reported that cases of calcitonin negative MTC, typically presenting in advanced stage, had high Ki67 levels and associated with poor prognosis (16). In a cross sectional study of 44 patients, calcitonin immunostaining was shown to be useful as a prognostic factor (17). Other retrospective studies show that in patients with virulent MTC, CEA expression in both primary and metastasis tissue remained but calcitonin staining was poor or absent (18). However, other studies reported that the prognosis of calcitonin negative MTC is heterogenous and weak immunostaining of calcitonin was not associated with aggressive behavior or poor prognosis (12,15). Our patient’s tumor was confined to thyroid tissue, with negative margins and no clinical suspicion of lymph node metastasis or any other worrisome features, and also had low Ki-67 expression, which supports non-aggressive behavior and better prognosis.
The explanation for low serum calcitonin in the setting of advanced MTC is thought to be tumor dedifferentiation (19) because calcitonin is a marker for terminal cellular differentiation of C-cells. This is usually accompanied by increased CEA level. Another explanation for this is the ‘Hook effect’, which has been reported for calcitonin measurement, whereby if the concentration of antigen is extremely high, the capture and the signal antibodies are saturated with the antigen, and thus, the antigen concentration measured is falsely low (20). However, neither explanation seems applicable in our case because the tumor had low Ki67 expression (<1%) and was limited to the thyroid gland with no other aggressive features. The lymph nodes were not found to be suspicious during physical examination or intra-operatively, although lymph node dissection was not done because the aim of operation was for diagnosis.
Since our patient’s personal and family history of cancer could be caused by a mutation in the MEN1 gene or mutation in one of the other susceptibility genes associated with neuroendocrine tumors, such as MLH1, MSH2, MSH6, PALB2, APC, MUTYH and CHEK2, genetic consultation and somatic gene testing were recommended. Sequencing, deletion, and duplication analysis of 130 genes, including BRCA1/2, KIT, MEN1, NF1, NF2, RET, SDHB and TERT were negative for mutations. This result significantly reduces the chance that the patient has an inherited predisposition cancer.
A previous study suggested that the prognosis of non-secretory MTC are markedly heterogenous and the poor prognosis patients were associated with high KI67 level and RET918 gene mutations (12). However, a report that included two cases with focally positive calcitonin on immunohistochemistry noted that one patient had no recurrent disease after 10 years of follow-up, and even suggested favorable prognosis in the patients with non or low calcitonin producing MTC (15). According to the revised ATA guidelines for the management of medullary thyroid carcinoma, MTC usually require total thyroidectomy plus central neck dissection. While, lateral neck dissection is only required when there is evidence of nodal metastasis (1). Although, it is important to mention that MTC is only surgical curable and it usually associated with aggressive behavioral, our patient’s tumor showed the opposite profile. Our patient’s tumor had low Ki67 expression and no RET mutation, suggesting non aggressive behavior of the tumor. Ultrasound neck pre-operatively also showed no evidence of suspicious lymph node. Therefore, we suggested either active observation or completion thyroidectomy for her.
Post-operative follow-up with calcitonin monitoring in patients with low expression of calcitonin can be difficult, as serum biomarkers are unreliable. Calcitonin level has been observed to rise in several cases of recurrent calcitonin-negative MTC. The reasons for this are unclear; nevertheless, authors still suggest using calcitonin as a follow up tool.
Routine imaging was also important in our case. The contralateral lobe had small benign-looking nodules which require follow up imaging. Routine follow up with neck ultrasound is an acceptable method for detecting recurrent nodes or new nodules in the thyroid remnant, but may fail to detect very small lesions. Some authors suggest FDG-PET/CT (16) but price and availability may be limitations. For our patient, follow up with ultrasound neck is planned.
In conclusion, our case suggests that limited calcitonin expression cannot completely exclude the diagnosis of MTC. This rare type of MTC should be differentiated from a typical MTC. The treatment and post-operative surveillance should be tailored based on individual tumor characteristics.
Acknowledgements
We would like to thank Pamela Derish M.A. who has provided the editorial support for our paper.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. [Crossref] [PubMed]
- Schmid KW, Ensinger C. “Atypical” medullary thyroid carcinoma with little or no calcitonin expression. Virchows Arch 1998;433:209-15. [Crossref] [PubMed]
- Parmer M, Milan S, Torabi A. Calcitonin-Negative Neuroendocrine Tumor of the Thyroid. Int J Surg Pathol 2017;25:191-4. [Crossref] [PubMed]
- Chernyavsky VS, Farghani S, Davidov T, et al. Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid 2011;21:193-6. [Crossref] [PubMed]
- Suzuki A, Hirokawa M, Takada N, et al. Fine- needle aspiration cytology for medullary thyroid carcinoma: a single institue experience in Japan. Endocrine Journal 2017;64:1099-104. [Crossref] [PubMed]
- Kudo T, Miyauchi A, Ito Y, et al. Diagnosis of Medullary Thyroid Carcinoma by Calcitonin Measurement in Fine Needle Aspiration Biopsy Specimens. Thyroid 2007;17:635-8. [Crossref] [PubMed]
- Trimboli P, Guidobaldi L, Bongiovanni M, et al. Use of fine-needle aspirate calcitonin to detect medullary thyroid carcinoma: A systematic review. Diagn Cytopathol 2016;44:45-51. [Crossref] [PubMed]
- Cibas ES, Ali SZ. Conference NCITFSotS. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol 2009;132:658-65. [Crossref] [PubMed]
- Nishino M. Molecular cytopathology for thyroid nodules: A review of methodology and test performance. Cancer Cytopathology 2016;124:14-27. [Crossref] [PubMed]
- Beaudenon-Huibregtse S, Alexander EK, Guttler RB, et al. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid 2014;24:1479-87. [Crossref] [PubMed]
- Cohen R, Campos JM, Salaun C, et al. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d'Etudes des Tumeurs a Calcitonine (GETC). J Clin Endocrinol Metab 2000;85:919-22. [Crossref] [PubMed]
- Frank-Raue K, Machens A, Leidig-Bruckner G, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid 2013;23:294-300. [Crossref] [PubMed]
- Redding AH, Levine SN, Fowler MR. Normal Preoperative Calcitonin Levels Do Not Always Exclude Medullary Thyroid Carcinoma in Patients with Large Palpable Thyroid Masses. Thyroid 2000;10:919-22. [Crossref] [PubMed]
- Sand M, Gelos M, Sand D, et al. Serum calcitonin negative medullary thyroid carcinoma. World J Surg Oncol 2006;4:97. [Crossref] [PubMed]
- Sama MT, Rossetto Giaccherino R, Gallo M, et al. Clinical challenges with calcitonin-negative medullary thyroid carcinoma. J Cancer Res Clin Oncol 2016;142:2023-9. [Crossref] [PubMed]
- Wang TS, Ocal IT, Sosa JA, et al. Medullary Thyroid Carcinoma without Marked Elevation of Calcitonin: A Diagnostic and surveillance Dilemma. Thyroid 2008;18:889-94. [Crossref] [PubMed]
- Saad MF, Ordonez NG, Guido JJ, et al. The prognostic value of calcitonin immunostaining in medullary carcinoma of the thyroid. J Clin Endocrinol Metab 1984;59:850-6. [Crossref] [PubMed]
- Mendelsohn G, Wells SA Jr, Baylin SB. Relationship of tissue carcinoembryonic antigen and calcitonin to tumor virulence in medullary thyroid carcinoma. An immunohistochemical study in early, localized and virulent disseminated stages of disease. Cancer 1984;54:657-62. [Crossref] [PubMed]
- Leboulleux S, Baudin E, Travagli JP, et al. Medullary thyroid carcinoma. Clin Endocrinol (Oxf) 2004;61:299-310. [Crossref] [PubMed]
- Leboeuf R, Langlois MF, Martin M, et al. "Hook effect" in calcitonin immunoradiometric assay in patients with metastatic medullary thyroid carcinoma: case report and review of the literature. J Clin Endocrinol Metab 2006;91:361-4. [Crossref] [PubMed]
Cite this article as: Sukpanich R, Khanafshar E, Suh I, Gosnell J. Case report of a neuroendocrine tumor of the thyroid gland with limited calcitonin expression: a diagnostic challenge. AME Case Rep 2019;3:12.


