
Solitary fibrous tumour of the parotid gland: a case report and a 15-year literature review
Introduction
First discovered in the pleura, solitary fibrous tumour (SFT) was previously falsely thought to be of mesothelial origin. It is then shown to be a fibroblastic mesenchymal neoplasm likely from adult mesenchymal stem cells hence giving rise to a variety of anatomical origins (1). SFTs in the head and neck region comprise about 6% of all SFTs in previously published data (2). Of the head and neck SFTs, the most common sites involved are the sinonasal tract and orbit, followed by oral cavity, then salivary glands (2). A review of literature found 40 reported cases of SFT in major salivary glands from 2004 to 2018. Here we report an additional case of SFT arising from the left parotid gland as well as a 15-year review of the literature on SFTs arising from major salivary glands.
Case presentation
A 51-year-old Chinese gentleman presented with a 3-month history of a left angle of mandible lump. Physical examination showed a left angle of mandible 2.5 cm firm mass that was non-tender, with no overlying skin changes. The facial nerve function was normal, and there were no other palpable lumps on examination of his neck.
Preoperative computed tomography (CT) scan of the neck showed a 4.2 cm × 2.9 cm× 3.3 cm fairly well marginated homogeneously enhancing lesion within the left parotid gland (Figure 1). There was no central necrosis or adjacent stranding. Fine needle aspiration cytology showed cells which appear basaloid, with minimal stroma suggestive of an aspirate from a basaloid lesion, including basal cell adenoma and a cellular pleomorphic salivary adenoma. The patient underwent a left subtotal parotidectomy. Post-operatively, the patient recovered uneventfully, and has recovered well from a partial left facial nerve paresis.
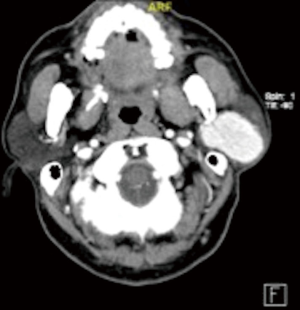
The excised specimen was a 6 cm lobulated fleshy tumour involving both superficial and deep lobes of the parotid gland, with epicentre likely in the deep lobe. The tumour lies lateral to the retromandibular vein but deep to the facial nerve.
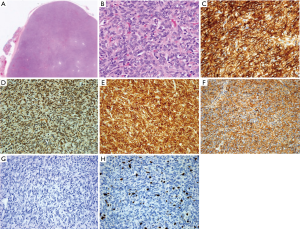
Figure 2 show the microscopic appearance of the tumour. The tumour is cellular with short fascicles of plump spindle cells with little intervening fibrous stroma, displaying a hemangiopericytomas pattern. There are no areas of de-differentiation. The margins were not infiltrated by tumour, although very close, focally <0.1 mm. The high power view show spindle cells displaying oval nuclei without any significant atypia. Immunohistochemical studies show that the neoplastic cells diffusely express CD34, bcl2 and CD99, and nuclear expression of STAT6. There was no expression of cytokeratins (AE1-3), CK14, SMA, CD21, CD35 or p63. The Ki-67 proliferation index was approximately 10–20%. Hence, a diagnosis of SFT was made.
The patient was discussed at a multi-disciplinary head and neck tumour board and decision was made to offer post-operative Radiotherapy. His tumour margins were considered to be close, and Ki 67 proliferation index was not low. He was followed-up for 3 months with no clinical evidence of recurrence. However, the patient declined adjuvant treatment.
Discussion
Most SFTs of the parotid origin reported in the literature are of the fibrous variant characterised by alternating areas of fibrous hypocellularity and hypercellularity composed of round-to-spindle cells, tapering cytoplasm with a fascicular, storiform arrangement (3,4). They are associated with “staghorn” vasculature.
It is also thought to be synonymous with Giant Cell Angiofibroma and Hemangiopericytomas. Since 2002, the terms Hemangiopericytoma and SFT had been unified under the term SFT according to WHO classification of soft tissue and bone tumors, and in 2013 the term Hemangiopericytoma has been replaced by SFT (4).
SFT of the major salivary gland are a rare group of spindle-cell tumours. We did a literature review in PubMed using the search terms “Hemangiopericytoma” and “Solitary Fibrous Tumour” in combination with “Sublingual Gand”, “Submandibular Gland”, “Parotid Gland” “Salivary Gland”. We selected articles published within the time period of January 2004 to October 2018. Clinical series of head and neck SFTs were included if critical information on salivary gland SFTs were reported. Foreign language articles were included only if an English version was available, and duplicated cases reported in multiple articles were excluded.
Including our case reported here, only 40 cases have been reported over the past 15 years from 2004 to 2018. Thirty-four of which were from the parotid gland (including our case), five were from submandibular gland and one from the sublingual gland. Table 1 summarises the characteristics of these reviewed cases (1,2,5-26).
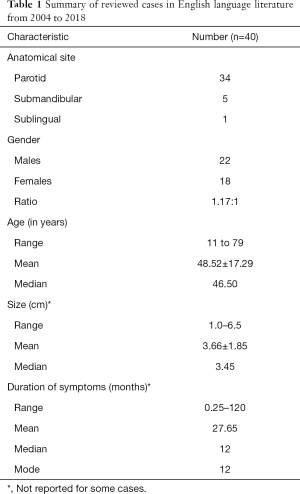
Full table
Clinical presentation and investigations
Majority of reported cases involve the parotid gland, rather than the submandibular and sublingual glands. The age range of patients with major salivary gland SFTs is very wide from 11 to 79 years (mean 48.52 years). Although SFTs are not known to have any gender predilection, our review showed a slightly higher incidence of SFTs in men (54%) as compared to women (46%). This is similar to findings in other studies done on head and neck SFTs (8).
SFT of the parotid and other major salivary glands usually present as a slow growing, palpable non-tender mass. Occasionally, they may cause compression symptoms such as dysphagia, in which case extension to the parapharyngeal space is suspected (27). Clinical presentation is similar to that of other tumors such as pleomorphic adenomas and lipomas. Patients complain of a firm and well-defined mass that is slow-growing over a period of months to years (Table 1). FNAC is rarely helpful. Radiographic findings are also nonspecific. Magnetic resonance imaging usually shows an isointense mass on T1-weighted images and high signal intensity with enhancement in T2-weighted images (18). Therefore, a diagnosis of SFT is made frequently based on histological and immunohistochemical analysis.
Histology and immunochemistry
Macroscopically, SFTs are known to be well-circumscribed, firm, white-tan or gray and encapsulated.
CD34 has been shown to be a very useful marker in the diagnosis of SFT. Only a portion of the cases found reported Immunohistochemical results, and the stains performed vary across literature. We subanalysed cases that had reported stainings for the three most common markers namely CD34, bcl-2 and CD99, summarised in Table 2.
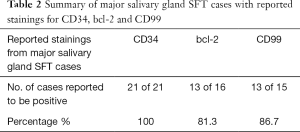
Full table
This high rate of positivity is consistent with studies done in SFTs of other anatomical locations (5,28,29) Bcl-2 may be positive in other kinds of mesenchymal tumors like sarcomas, hence are not as specific for SFT (8). In some cases of malignant and dedifferentiated SFT, CD34 may be negative. Hence, CD34 is useful in combination with Bcl-2 as a consistent characteristic of SFT (29).
Immunoreactivity to STAT6 has been shown to be highly sensitive and specific for SFTs (28). This finding is useful, as less than 10% of other spindle cell tumors are positive for STAT6 (29). In our case, STAT6 was used as an adjunct to diagnosis and found to be positive. Although none of the other salivary gland SFTs in our review were tested for STAT6, a multi-institutional clinicopathological study on SFT in other anatomical sites found that all 45 out of 45 (100%) cases showed positivity to STAT6 (28). Yoshida et al. also published a validation study where also 49 out of 49 (100%) SFTs showed STAT6 expression, and only a 2.5% (4 out of 155) of cases of other tumor mimics of SFT showed weak nuclear STAT6 expression (30).
Treatment and outcome
Complete surgical resection is the prevailing treatment, and most authors recommend long-term follow-up for all patients with SFT due to the possibility of recurrence and a minority behaving aggressively (31). The pathological features used to predict poorer outcomes in SFT tumors of the head and neck region are hypercellularity, nuclear atypia, more than 5 mitoses per 10 high-power fields and tumour necrosis (2,4). These features may not be the sole predictor of behaviour as some SFTs that do not meet the above criteria are shown to have recurred. Although reported in a minority of SFT involving other anatomical sites, metastases have not yet to be reported in SFTs of the head and neck region (31).
There were two cases of Parotid SFTs in 2014 and 2015 that reported recurrence at 24 and 20 months post-surgery respectively (6,8). Involved margins was only reported in the latter case. Ki 67 proliferation rate of <4% is generally considered benign, and >20% considers the tumour a malignant variant (8,32). However, in these two cases, Ki67 rate was reported as 5% and 5–10% respectively, a range that is considered intermediate. In both cases, adjuvant radiotherapy was not administered.
Ganly et al. (33) suggest that patients with positive surgical margins or whose tumors have a malignant component benefit from adjuvant postoperative radiotherapy. However, there are few reports concerning adjuvant treatment and their follow up periods are of short duration. Longer term studies in this area may be beneficial.
Conclusions
In summary, this case study reports a rare case of solitary fibrous tumour in the parotid gland occurring in a 51-year-old Chinese gentleman. Diagnosis of this tumour was made mainly based on histology and immunohistochemical staining namely CD34 used in combination with Bcl-2 and STAT6. Its presentation, epidemiology and size is consistent with the ranges reported in literature of other SFTs of the major salivary glands over the past 15 years. Complete surgical excision is the most widely accepted treatment, with a lack of information surrounding the efficacy of radiotherapy. Hence, SFTs occurring in the major salivary glands should be followed-up with care considering their unpredictable course and potential for recurrence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Bauer JL, Miklos AZ, Thompson LDR. Parotid gland solitary fibrous tumor: a case report and clinicopathologic review of 22 cases from the literature. Head Neck Pathol 2012;6:21-31. [Crossref] [PubMed]
- Smith SC, Gooding WE, Elkins M, et al. Solitary Fibrous Tumors of the Head and Neck: A Multi-Institutional Clinicopathologic Study. Am J Surg Pathol 2017;41:1642-56. [Crossref] [PubMed]
- Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology 2006;48:63-74. [Crossref] [PubMed]
- Organization WH. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013. 468 p.
- Sousa AA, Souto GR, Sousa IA, et al. Solitary fibrous tumor of the parotid gland: Case report. J Clin Exp Dent 2013;5:e208-211. [Crossref] [PubMed]
- Alonso-Rodríguez E, González-Otero T, Castro-Calvo A, et al. Parotid gland solitary fibrous tumor with mandibular bone destruction and aggressive behavior. J Clin Exp Dent 2014;6:e299-302. [Crossref] [PubMed]
- Kwok MM, Subramaniyan M, Chan SW. Solitary Fibrous Tumour of the Parotid Gland: A Case Report and Review of the Literature. Case Rep Otolaryngol 2015;2015:741685. [Crossref] [PubMed]
- Künzel J, Hainz M, Ziebart T, et al. Head and neck solitary fibrous tumors: a rare and challenging entity. Eur Arch Otorhinolaryngol 2016;273:1589-98. [Crossref] [PubMed]
- Rais M, Kessab A, Sayad Z, et al. Solitary fibrous tumor occurring in the parotid gland: a case report. BMC Clin Pathol 2017;17:22. [Crossref] [PubMed]
- Chen D, Xuan J, Sun M, et al. Lipomatous hemangiopericytoma (adipocytic variant of solitary fibrous tumor) of the parotid gland: A case report and review of the literature. Oncol Lett 2013;6:1380-2. [Crossref] [PubMed]
- Martínez Vecina V, Zarraonandia I, Monzón M, et al. Solitary fibrous tumour of the submandibular gland. Acta Otorrinolaringol Esp 2015;66:302-4. [Crossref] [PubMed]
- Shi W, Wei Z. Solitary fibrous tumor of the submandibular region. Oncol Lett 2015;9:984-6. [Crossref] [PubMed]
- Chis O, Albu S. Giant solitary fibrous tumor of the parotid gland. Case Rep Med 2014;2014:950712. [Crossref] [PubMed]
- Thompson M, Cheng LHH, Stewart J, et al. A paediatric case of a solitary fibrous tumour of the parotid gland. Int J Pediatr Otorhinolaryngol 2004;68:481-7. [Crossref] [PubMed]
- Cho KJ, Ro JY, Choi J, et al. Mesenchymal neoplasms of the major salivary glands: clinicopathological features of 18 cases. Eur Arch Otorhinolaryngol 2008;265 Suppl 1:S47-56. [Crossref] [PubMed]
- Messa-Botero OA, Romero-Rojas AE, Chinchilla Olaya SI, et al. Primary malignant solitary fibrous tumor/hemangiopericytoma of the parotid gland. Acta Otorrinolaringol Esp 2011;62:242-5. [Crossref] [PubMed]
- Suárez Roa ML, Ruíz Godoy Rivera LM, Meneses García A, et al. Solitary fibrous tumor of the parotid region. Report of a case and review of the literature. Med Oral 2004;9:82-8. [PubMed]
- Kim HJ, Lee HK, Seo JJ, et al. MR imaging of solitary fibrous tumors in the head and neck. Korean J Radiol 2005;6:136-42. [Crossref] [PubMed]
- Ridder GJ, Kayser G, Teszler CB, et al. Solitary fibrous tumors in the head and neck: new insights and implications for diagnosis and treatment. Ann Otol Rhinol Laryngol 2007;116:265-70. [Crossref] [PubMed]
- Takahama A, León JE, de Almeida OP, et al. Nonlymphoid mesenchymal tumors of the parotid gland. Oral Oncol 2008;44:970-4. [Crossref] [PubMed]
- Manglik N, Patil S, Reed MF. Solitary fibrous tumour of the parotid gland. Pathology 2008;40:89-91. [Crossref] [PubMed]
- Cristofaro MG, Allegra E, Giudice M. Two new localizations of solitary fibrous tumor in the italian population: parotid gland and oral cavity-review of the literature. J Oral Maxillofac Surg 2012;70:2360-7. [Crossref] [PubMed]
- Garg D, Palaskar S, Shetty VP, et al. Diagnostic dilemma: solitary fibrous tumor or hemangiopericytoma of the submandibular region in a patient with multiple odontogenic keratocysts. J Pediatr Oncol Nurs 2009;26:136-41. [Crossref] [PubMed]
- Ayad T, Ghannoum J. Solitary fibrous tumor with pseudo-lipoblasts involving the sublingual gland: report of a case and review of the literature. Eur Arch Otorhinolaryngol 2007;264:93-8. [Crossref] [PubMed]
- Liu Y, Tao X, Shi H, et al. MRI findings of solitary fibrous tumours in the head and neck region. Dentomaxillofac Radiol 2014;43:20130415. [Crossref] [PubMed]
- Oktem F, Karaman E, Mamak A, et al. Hemangiopericytoma of the parotid gland: a case report. Kulak Burun Bogaz Ihtis Derg 2007;17:112-5. [PubMed]
- Sato J, Asakura K, Yokoyama Y, et al. Solitary fibrous tumor of the parotid gland extending to the parapharyngeal space. Eur Arch Otorhinolaryngol 1998;255:18-21. [Crossref] [PubMed]
- Tai HC, Chuang IC, Chen TC, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol 2015;28:1324-35. [Crossref] [PubMed]
- Geramizadeh B, Marzban M, Churg A. Role of Immunohistochemistry in the Diagnosis of Solitary Fibrous Tumor, a Review. Iran J Pathol 2016;11:195-203. [PubMed]
- Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 2014;38:552-9. [Crossref] [PubMed]
- Ito H, Fukuda M, Imamura Y, et al. A malignant solitary fibrous tumor in the retroperitoneum. Int J Clin Oncol 2008;13:173-5. [Crossref] [PubMed]
- Meyer M, Krause U. Solitary fibrous tumors of the pleura. Chirurg 1999;70:949-52. [Crossref] [PubMed]
- Ganly I, Patel SG, Stambuk HE, et al. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg 2006;132:517-25. [Crossref] [PubMed]
Cite this article as: Lim DW, Tan TS, Tan JL, Venkateswaran K. Solitary fibrous tumour of the parotid gland: a case report and a 15-year literature review. AME Case Rep 2019;3:14.


