
A rare case of acute pancreatitis due to very severe hypertriglyceridemia treated with subcutaneous insulin and lipid lowering drugs
Introduction
The diagnosis of acute pancreatitis in a patient requires the presence of two of the following three criteria: (I) acute onset of persistent, severe; (II) epigastric pain often radiating to the back, elevation in serum lipase or amylase to three times or greater than the upper limit of normal; (III) characteristic radiographic evidence (1). Hypertriglyceridemia is a potential cause of acute pancreatitis when levels are greater than 1,000 mg/dL (2). Very severe hypertriglyceridemia is classified as levels above 2,000 mg/dL. Management includes targeting the pancreatitis with intravenous fluids, pain control, and nutritional support. While apheresis with therapeutic plasma exchange is a known option for severe hypertriglyceridemia, we present a rare case with management with intravenous fluids, subcutaneous insulin, statins, and fibrates in a patient with a triglyceride level of 12,234 mg/dL who presented with severe epigastric pain radiating to her back.
Case presentation
The patient is a 45-year-old morbidly obese Hispanic female with a past medical history of hypertension, diabetes mellitus type 2, and gastroesophageal reflux disease, who presented to the emergency department with complaints of severe 10/10 epigastric pain for approximately 8 h. The patient was in her normal state of health until she felt this sharp, stabbing, epigastric abdominal pain that radiates to her back. She endorsed four episodes of non-bilious, non-bloody vomiting. The pain was partially alleviated by over the counter ibuprofen. The patient’s last meal was 2 h before the abdominal pain began in which she ate homemade frijoles. The patient had a laparoscopic cholecystectomy approximately two years prior to presentation. The patient denies any alcohol or drug usage. Physical exam was only significant for tender to light palpation in the epigastric abdominal region; otherwise, bowel sounds were active, the abdomen was non-distended and soft. On initial laboratory workup, the patient had a lipase significant for 14, 923 U/L and triglycerides level of 12,234 mg/dL. Of note, the patient has uncontrolled diabetes mellitus with a hemoglobin A1c of 9.7% and random blood glucose of 382 mg/dL. Furthermore, the initial chemistry panel demonstrated albumin level 3.7 gm/dL, a corrected calcium level of 8.0 mg/dL. Lactic acid levels obtained and were 1.1. Ultrasound of the gallbladder was done upon admission and showed no evidence of biliary stones. The patient was kept nil per os (NPO), started on 250 mL/h lactated Ringer’s solution, and pain control with intravenous morphine. The patient was started on insulin glargine 10 units in the morning, 10 units at night as well as insulin lispro as a high correction scale with meals. On hospital day 2, the patient spiked a temperature of 101.4 °F and continued to endorse epigastric pain. Chemistry panel albumin level of 2.8, a corrected calcium level of 9.0. Repeat lactic acid levels were found to be 0.6. A computed tomography of the chest, abdomen, and pelvis with contrast demonstrated grade E pancreatitis without evidence of necrosis. After approximately 48 h, the patient was additionally started on rosuvastatin 20 milligrams and fenofibrate 160 milligrams. The patient was starting to tolerate clear liquid diet. The patient’s hyperglycemia on hospital day three continued to be poorly controlled and insulin glargine was increased to 15 units. A new triglyceride level on hospital day 4 revealed 1,824 mg/dL and the patient reported the epigastric pain was a 2/10 and was tolerating a full liquid diet. Chemistry panel demonstrated, albumin level of 2.3, a corrected calcium level of 8.9. Repeat lactic acid levels continued to demonstrate a downward trend at less than 0.4 and further laboratory evaluation of lactic acid was deemed unnecessary and discontinued.
On hospital day 5 the patient endorsed constant abdominal pain with radiation to the back began to have a cyclic fever with a Tmax of 101.3 °F and piperacillin/tazobactam was restarted as the patient continued to have leukocytosis of 14.1 and bandemia of 24% and rosuvastatin was increased to 40 mg. A repeat computed tomography of the chest, abdomen, and pelvis demonstrated an interval increase in the peripancreatic fluid with extension of the fluid into the left iliac fossa. The patient was advanced to a soft diet as her liquid diet was well tolerated and the patient was showing improvement in epigastric pain.
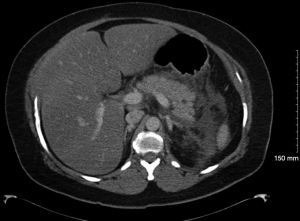
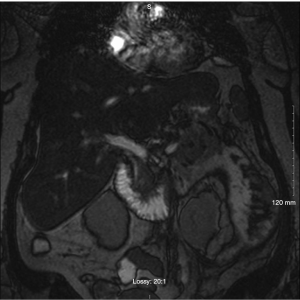
On hospital day 8, the patient spiked a temperature with a Tmax 101.3 °F and complete blood count (CBC) with differential demonstrated up-tending leukocytosis with a white blood cell (WBC) of 18.0, 14.1 polymorphonuclear neutrophils (PMNs) and 10% bands while being given piperacillin/tazobactam it was deemed necessary to discontinue piperacillin/tazobactam start meropenem 1g IV Q8H. A repeat lipid panel demonstrated a decrease in triglycerides to 465. A repeat computed tomography of the chest, abdomen, and pelvis with contrast demonstrated moderate left pleural effusion that was shown to be increased from previous studies, trace amounts of free fluid in the right pleural space demonstrating interval regression, multiple pockets of peripancreatic, perigastric and perisplenic collections extending along Gerota’s fascia, into the left paracolic gutter and in continuation with the left adnexa unchanged in size and extent but demonstrating a more conspicuous peripheral wall enhancement on the current exam indicative of developing abscesses versus pseudocysts (Figure 1). Interventional radiology and general surgery were consulted for the possibility of incision and drainage of the abscess but it was deemed an unnecessary risk due to the small amount of fluid accumulation and the risk of adverse complications. The patient was placed NPO and restarted on maintenance intravenous fluids. A magnetic resonance cholangiopancreatography (MRCP) was obtained and demonstrated focal narrowing of the common hepatic duct in the portal region with suggestion of a 3 mm lesion adherent to the duct wall causing compression. Moderate peripancreatic fluid collections, including in the retroperitoneal, pararenal, paracolic gutter, and mesenteric regions which were primarily on the left side all of which are findings consistent with pancreatitis (Figure 2). On hospital day 10 diet was advanced to ice chips and clear liquid and insulin glargine was increased from 15 units BID to 20 units BID. On hospital day 11, the patient remained afebrile for 24 h and patients’ diet was advanced from clear liquid to consistent carb with 9 tablets pancrelipase with each meal (500 units/kg/meal). The patient continued to remain afebrile, tolerate per os (PO) diet and IV fluid administration was discontinued. The patient remained afebrile, was tolerating a full diet and was discharged on long-term fenofibrate and statin therapy, levofloxacin, metronidazole, as well as counseled on compliance with her other medications to control her diabetes and hypertension. After ruling out other competing etiologies, a final diagnosis of hypertriglyceridemia-induced acute pancreatitis was made.
Discussion
Although rare, hypertriglyceridemia is the third leading cause of acute pancreatitis after gallstones and alcohol abuse. Different classifications of hypertriglyceridemia exist ranging from mild, moderate, severe, and very severe. Hypertriglyceridemia is considered a significant risk for acute pancreatitis when levels are greater than 1,000 mg/dL which is the cutoff classified as severe (2). In our case, we present a patient with very severe (>2,000 mg/dL) hypertriglyceridemia; furthermore, we have a very rare case in which the triglycerides are even greater than 10,000 mg/dL which has been rarely been reported in the literature. Our patient had a triglyceride level of 12,234 mg/dL with acute epigastric pain and a severely elevated lipase level of 14,923. Subsequent lipase levels showed a downward trend to 592, and 363 on the 3rd and 10th days following the patient’s recovery. Although our patient initially presented with mild hypocalcemia, this quickly resolved with administration calcium gluconate. Lactic acid levels were obtained for our patient upon presentation and were found to be insignificant and continued to trend down on subsequent laboratory evaluations until it was deemed unnecessary to continue.
The most common causes of hypertriglyceridemia-induced acute pancreatitis include lipid disorders, alcohol abuse, medication adverse effects, or poorly controlled diabetes mellitus. In our case, the patient had a known history of poorly-controlled diabetes mellitus with the most recent hemoglobin A1C as 9.7%. Family history is an important risk factor in the pathogenesis; however, our patient had no predisposing genetic risk factors or a known family history of lipid disorders.
The pathogenesis of elevated triglycerides causing acute pancreatitis involves the breakdown of triglyceride into toxic free fatty acids by the pancreatic lipases which is the actual underlying cause of lipotoxicity during acute pancreatitis (3,4). The severity of the damage is twofold as there is an inflammatory response caused by pancreatitis as well as the injury caused by lipotoxicity from triglyceride hydrolysis. The combination of very severe hypertriglyceridemia, elevated lipase levels, and elevated free fatty acid levels can be further complicated by systemic inflammation from acute pancreatitis, direct activation of toll-like receptor 2 and toll-like receptor 4 by free fatty acids, and direct lipotoxicity (3,5). Our patient had severely elevated hypertriglyceridemia and lipase levels, eventually leading to systemic inflammation and development of hyperthermia and continued epigastric pain despite pain medications and acetaminophen.
Initial management targets to prevent further symptoms by targeting treatment toward acute pancreatitis as well as reducing serum triglyceride levels in order to prevent the development of necrotizing pancreatitis and organ failure. For acute pancreatitis treatment, we aimed at the three fundamental goals of NPO, high volume intravenous fluids, and pain control. The standard of care in patients with hypertriglyceridemia-induced acute pancreatitis with worrisome features including but not limited to hypocalcemia, elevated temperature, elevated WBC count is plasmapheresis and if not feasible or available, then intravenous insulin is to be administered (6-8). Management of acute pancreatitis typically involves NPO, to allow the inflammation of the pancreatitis to subside. Our patient, however, wanted to resume her regular diet after one day of clear liquids. Studies have shown that patients who were able to tolerate oral intake and were started on regular diet showed faster recovery time compared to patients who waited instead without any adverse effects (9-11). Ideally, treatment by inhibition of the pancreatic secretions would stop the inflammation of the pancreas and allow for recovery, but despite knowledge of the physiology of pancreas and its secretions, there is no pharmacological treatment for control of pancreatic enzyme release. One study showed that octreotide, a somatostatin analogue, was only able to inhibit the release of one pancreatic enzyme—amylase persistently, showing no effects in levels of trypsin or chymotrypsin secretions (12). Despite our patient’s initial presentation of hypocalcemia, this was quickly corrected and lactic acid levels were never demonstrated significant trends in elevation it was decided that our management comprise NPO, high volume intravenous fluids, pain control, subcutaneous insulin regimen, and lipid-lowering therapy with a statin and a fibrate. The patient’s pain was well controlled, triglyceride levels decreased gradually, and the patient was discharged in stable condition with her home medications along with the addition of long-term statin and fibrate therapy.
Despite the positive outcome in this case, our patient’s hospital course was complicated by bibasilar pleural effusions, multiple abdominal fluid collections, cyclical fevers, and continued abdominal pain. It can be safely assumed that our more conservative approach to the management of this case played a role in the protracted hospital course for this patient and adherence to the accepted standard of care including plasmapheresis or at least IV insulin administration would have led to a shorter and less complicated hospital course as well as reduce the overall cost of treatment for this patient.
Conclusions
Hypertriglyceridemia is a potential cause of acute pancreatitis. The diagnosis is established with basic history, physical, and a lipid panel. We present a case to support the established treatment guidelines for the management of very severe hypertriglyceridemia acute pancreatitis as either plasmapheresis or IV insulin as our patient with very severe hypertriglyceridemia-induced acute pancreatitis that was managed with NPO, high volume intravenous fluids, pain control, subcutaneous insulin, a statin, and a fibrate suffered from a protracted and complicated hospital course that might have otherwise been avoided.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2969-89. [Crossref] [PubMed]
- Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 2011;3:107ra110. [Crossref] [PubMed]
- Yang F, Wang Y, Sternfeld L, et al. The role of free fatty acids, pancreatic lipase and Ca+ signalling in injury of isolated acinar cells and pancreatitis model in lipoprotein lipase-deficient mice. Acta Physiol (Oxf) 2009;195:13-28. [Crossref] [PubMed]
- Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol 2008;14:4558-61. [Crossref] [PubMed]
- Ipe TS, Pham HP, Williams LA 3rd. Critical updates in the 7th edition of the American Society for Apheresis guidelines. J Clin Apher 2018;33:78-94.
- Jabbar MA, Zuhri-Yafi MI, Larrea J. Insulin therapy for a non-diabetic patient with severe hypertriglyceridemia. J Am Coll Nutr 1998;17:458-61. [Crossref] [PubMed]
- Mikhail N, Trivedi K, Page C, et al. Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin. Am J Emerg Med 2005;23:415-7. [Crossref] [PubMed]
- Eckerwall GE, Tingstedt BB, Bergenzaun PE, et al. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery--a randomized clinical study. Clin Nutr 2007;26:758-63. [Crossref] [PubMed]
- Moraes JM, Felga GE, Chebli LA, et al. A full solid diet as the initial meal in mild acute pancreatitis is safe and result in a shorter length of hospitalization: results from a prospective, randomized, controlled, double-blind clinical trial. J Clin Gastroenterol 2010;44:517-22. [PubMed]
- Vaughn VM, Shuster D, Rogers MAM, et al. Early Versus Delayed Feeding in Patients With Acute Pancreatitis: A Systematic Review. Ann Intern Med 2017;166:883-92. [Crossref] [PubMed]
- Friess H, Bordihn K, Ebert M, et al. Inhibition of pancreatic secretion under long-term octreotide treatment in humans. Digestion 1994;55 Suppl 1:10-5. [Crossref] [PubMed]
Cite this article as: Bajaj T, Grandhe S, Duong H, Ratnayake SN. A rare case of acute pancreatitis due to very severe hypertriglyceridemia treated with subcutaneous insulin and lipid lowering drugs. AME Case Rep 2019;3:26.


