
Splenic infarction secondary to myelodysplastic syndrome: unravelling more etiologies
Introduction
Myelodysplastic syndrome (MDS) is a clonal hematopoietic malignancy affecting the older adults due to several acquired heterogenous somatic mutations (1). Rarely, inherited familial syndromes are associated with early onset MDS. The incidence of MDS increases with age, and the clinical presentation varies widely depending upon the cellular lineage affected (1). Despite the clonal process resulting in hypercellular BM in MDS, patients usually present with peripheral cytopenia due to intramedullary apoptosis of dysplastic clonal cells (2). While infections, bleeding, and anemia are the most common findings in MDS, the other unusual findings are hepatomegaly, splenomegaly, lymphadenopathy (1). Although splenomegaly is rare, it is associated with several complications in MDS patients. Bourgeois et al. suggested that in some cases of low-risk MDS patients with splenomegaly, a certain extent of thrombocytopenia is attributed to peripheral destruction. Splenectomy is suggested to improve prognosis in these patients (3). According to Shimomura et al., enlarged spleen is associated with poor survival after allogenic stem cell transplant in MDS patients (4). Even though enlarged spleen causes the mentioned complications, splenic infarction or splenic rupture are never attributed till date in MDS cases. Here, we report a very first case of splenic infarction in an MDS patient with multilineage dysplasia and massive splenomegaly.
Case presentation
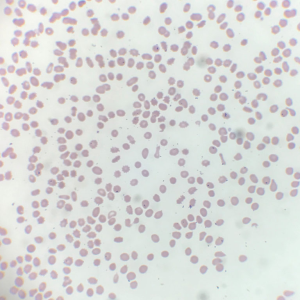
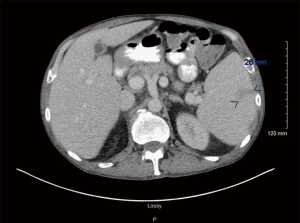
A 59-year-old Caucasian male presented with overnight events of high-grade fever, abdominal pain, and worsening fatigue; preceded by increased urinary frequency and dysuria for 2–3 days. The abdominal pain is a constant dull ache in suprapubic area. His medical history was significant for seizure disorder, recent diagnosis of untreated hepatitis C and MDS with multilineage dysplasia. Hepatitis C viral titres one month prior to this admission were 15,560 IU/L. Review of systems was negative for ulcers, purpura, rashes, arthralgias, sore throat, tingling, numbness, muscle weakness. He had no history of smoking, thalidomide or erythropoietin use. His medication list included tamsulosin, phenytoin, and lamotrigine. Physical exam was notable for temperature 101.8, hepatosplenomegaly with tenderness in suprapubic area. Laboratory values demonstrated pancytopenia with hemoglobin 7.1 (12.5–17.0 g/dL), leukocytes 1.2×103 (3.9–11.0 ×103/µL) and platelet 16×103 (150–450×103/µL). Coagulation studies showed prothrombin time (PT) 12.4 (9.1–12.0 sec), international normalized ratio (INR) 1.2 (2.0–3.5), activated partial thromboplastin time (APTT) 35.6 (25–35 sec). Liver function tests including synthetic function were normal. Peripheral blood smear (Figure 1) showed few elliptocytes, polychromatic red cells and teardrops but no significant hemoglobinopathies or atypical lymphocytes. Urine analysis was positive for nitrates and leukocyte esterase. Urine cultures showed pan-sensitive E. coli. For which, he was treated with cefepime. During the course of hospital stay, his urinary symptoms resolved but he developed new-onset non-radiating sharp pain in left upper quadrant (LUQ) of abdomen. Computed tomography (CT) abdomen with intravenous (IV) contrast (Figure 2) showed splenomegaly measuring up to 19 cm, and an ill-defined peripheral wedge-shaped hypodensity is seen in the mid pole of the spleen measuring up to 2.6 cm, highly characteristic of splenic infarction. No features of cirrhosis or vasculature abnormalities were noted. Further evaluation of splenic infarction was negative for anticardiolipin antibodies, human immunodeficiency virus (HIV), factor V Leiden mutation, factor II mutation, and Protein C & S deficiency. Echocardiogram showed no abnormal septal or valvular lesions and no evidence of thrombus or vegetation. As the suspicion for cryoglobulinemia was low due to lack of clinical features (rash, arthralgias, myalgias), further investigation was not pursued. Supportive medical management (fluids, ibuprofen) was provided due to the small size of the infarct and stable hemodynamic status. He received 7 units of packed red blood cell (PRBC) and 1 unit of platelet transfusion along with a dose of filgrastim for pancytopenia during the hospital stay. His fevers and LUQ pain gradually subsided without any surgical intervention.
Discussion
Splenomegaly is usually prevalent in indolent lymphomas, myelofibrosis, Gaucher disease, chronic myeloid leukemia (CML) and malarial splenomegaly syndrome (5) but unusual in MDS. Splenomegaly in neoplasms like myelofibrosis is due to extramedullary hematopoiesis (EMH) and abnormal migration of clonal hematopoietic cells from bone marrow (BM) to spleen due to dysregulated BM microenvironment (6). However, the pathogenesis of splenomegaly in MDS is unclear. While Kraemer et al. study showed that the splenomegaly is due to EMH (7), Kraus et al. showed that the splenomegaly in MDS is from the sequelae of dyspoiesis rather than proliferation (8).
Hematologic disorders causing splenomegaly also increase the risk for splenic infarction (9). Splenic infarction in these disorders is attributed to thromboembolic phenomenon or vascular congestion or oxygen supply-demand mismatch from splenomegaly and ongoing anemia etc. (10). Smith et al. suggested an increased risk in thrombotic events in MDS patients with concurrent use of erythropoietin stimulating agents and chemotherapeutic agents like thalidomide or lenalidomide (11). As a result, reports of deep vein thrombosis (DVT), pulmonary embolism (PE), or cerebral infarction were previously mentioned in MDS patients, but splenic infarction was never reported (12,13). Due to the lack of risk factors (lenalidomide use along with erythropoietin) and absence of vasculature abnormalities on CT abdomen with IV contrast, other probable etiologies were entertained. Splenic infarction in neoplasms like myelofibrosis is due to infiltration of splenic parenchyma by EMH and resulting vascular congestion (14). While in CML, it is attributed to decreased oxygen supply and increased oxygen requirement from anemia and splenomegaly respectively (10). As splenic infarction hasn’t been reported in MDS patients so far, no theory has been postulated explaining the underlying pathophysiology. We believe that an imbalance in demand and supply of oxygen from anemia, splenomegaly, and infection likely resulted in splenic infarction in our patient. Most of the patients with splenic infarction are clinically occult. In symptomatic patients, LUQ pain is the most common presenting complaint (5). Some less noted symptoms are fever, nausea, vomiting, and hemorrhagic shock (15). Nores et al. suggested that splenic infarction should be suspected in patients with known hematologic or thromboembolic conditions who develop LUQ pain and signs of localized or systemic inflammation (9). Although splenic infarction atypically presents with fever, we recommend ruling out infectious etiology before attributing the fever episodes to splenic infarction; especially in immunosuppressed patients.
The diagnosis of splenic infarction is usually based on radiologic imaging findings (5). The hypodense lesion seen in the mid pole of spleen on CT abdomen with contrast suggested several differentials like splenic infarction, splenic abscess, lymphoma etc. (16). But, the characteristic features of ill-defined wedge-shaped lesion with no contrast enhancement (including no rim enhancement), as noted in our patient, is diagnostic of splenic infarction (16). Splenic infarction is usually treated with supportive medical management like analgesics, hydration etc. In cases of hemodynamic instability or presence of complications, splenectomy is indicated (5). As our patient presented with hemodynamic stability, small sized splenic infarct and no complications, supportive medical management was offered without any need for splenectomy.
Conclusions
Splenic infarction is secondary to multiple pathological processes. But, MDS has never been mentioned before in literature until now. We hypothesize that the acute infection precipitated the splenic infarction due to oxygen demand-supply mismatch, along with the existing splenomegaly and anemia. Currently, the pathogenesis of splenomegaly remains unclear with different authors postulating various mechanisms. Further studies are needed to completely understand the mechanism of splenomegaly and splenic infarction in MDS. Also, enlarged spleen and its consequences on prognosis in MDS patients should be assessed further.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med 2009;361:1872-85. [Crossref] [PubMed]
- Clark DM, Lampert IA. Apoptosis is a common histopathological finding in myelodysplasia: the correlate of ineffective haematopoiesis. Leuk Lymphoma 1990;2:415-8. [Crossref] [PubMed]
- Bourgeois E, Caulier MT, Rose C, et al. Role of splenectomy in the treatment of myelodysplastic syndromes with peripheral thrombocytopenia: a report on six cases. Leukemia 2001;15:950-3. [Crossref] [PubMed]
- Shimomura Y, Hara M, Katoh D, et al. Enlarged spleen is associated with low neutrophil and platelet engraftment rates and poor survival after allogeneic stem cell transplantation in patients with acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol 2018;97:1049-56. [Crossref] [PubMed]
- Chapman J, Azevedo AM. Splenomegaly. Treasure Island (FL): StatPearls, 2019.
- Song MK, Park BB, Uhm JE. Understanding splenomegaly in myelofibrosis: association with molecular pathogenesis. Int J Mol Sci 2018.19. [PubMed]
- Kraemer D, Rüdiger T, Reimer P, et al. Splenectomy in patients with mixed myelodysplastic/myeloproliferative disease. Ann Hematol 2002;81:308-11. [Crossref] [PubMed]
- Kraus MD, Bartlett NL, Fleming MD, et al. Splenic pathology in myelodysplasia: a report of 13 cases with clinical correlation. Am J Surg Pathol 1998;22:1255-66. [Crossref] [PubMed]
- Nores M, Phillips EH, Morgenstern L, et al. The clinical spectrum of splenic infarction. Am Surg 1998;64:182-8. [PubMed]
- Parikh M, Geibel J. Splenic infarct. Emedicine, 2014.
- Smith SW, Sato M, Gore SD, et al. Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica 2012;97:15-20. [Crossref] [PubMed]
- Arai N, Nakata M, Morishita T, et al. Nihon Kyobu Shikkan Gakkai Zasshi 1992;30:468-71. [Pulmonary thromboembolism associated with myelodysplastic syndrome]. [PubMed]
- Yang X, Brandenburg NA, Freeman J, et al. Venous thromboembolism in myelodysplastic syndrome patients receiving lenalidomide: results from postmarketing surveillance and data mining techniques. Clin Drug Investig 2009;29:161-71. [Crossref] [PubMed]
- Mesa RA, Li CY, Schroeder G, et al. Clinical correlates of splenic histopathology and splenic karyotype in myelofibrosis with myeloid metaplasia. Blood 2001;97:3665-7. [Crossref] [PubMed]
- Cheng C-H, Bair M-J. Spontaneous splenic infarction as an uncommon cause of fever in a cirrhotic patient. Int J Gerontol 2017;11:121-4. [Crossref]
- Karlo CA, Stolzmann P, Do RK, et al. Computed tomography of the spleen: how to interpret the hypodense lesion. Insights Imaging 2013;4:65-76. [Crossref] [PubMed]
Cite this article as: Nalluru SS, Jindal V, Piranavan P, Kate Y, Siddiqui AD. Splenic infarction secondary to myelodysplastic syndrome: unravelling more etiologies. AME Case Rep 2019;3:31.


