
Excellent platinum dependent response to chemotherapy after relapse under TKI treatment in NSCLC with sensitizing EGFR mutations and no detectable resistance mutations: three case studies
Introduction
First-line tyrosine-kinase inhibitor (TKI) treatment is the current standard for patients with metastasized non-small cell lung cancer (NSCLC) and sensitizing epidermal growth factor receptor (EGFR) mutations being superior to first line chemotherapeutic treatment (1-3). Despite good initial responses, patients treated with TKIs relapse after an average of 12 months (4). About half of them develop known resistance mechanisms, e.g., T790M mutation, cMET-amplification or -skipping mutations or HER2-amplifications and therefore are qualified for further targeted therapies (5). However, many patients still have to be treated with standard chemotherapeutic regimens as checkpoint inhibition revealed to be not effective in EGFR mutated patients.
In this case series we describe three patients with similar progression patterns under TKI treatment in the absence of treatable resistance mechanisms and excellent platinum dependent response to chemotherapy and review the current literature focusing on effectiveness of standard chemotherapy in this cohort.
Patients
Case 1
A 37-year-old male patient presented with an adenocarcinoma of the lung with extensive brain and bone metastases and an activating EGFR mutation (Exon 21, L858R). As the brain metastases were symptomatic with headache and dizziness a whole brain radiation therapy was performed. After that afatinib treatment was initiated with 40 mg daily. The first staging showed a good tumor response. Four months later the patient developed dyspnea and pain due to a new hemorrhagic, malignant pleural effusion in combination with massive pulmonary embolism. Re-biopsy and next generation sequencing revealed the known L858R mutation, but no other resistance mutations. Subsequently, chemotherapy with cisplatin and pemetrexed was started. After the first cycle no further thoracentesis was needed, dyspnea and pain improved and the staging after the second cycle revealed a nearly complete remission (Figure 1A). Following six cycles of chemotherapy maintenance treatment with pemetrexed was subsequently continued. After a few weeks the patient showed a local tumor recurrence as well as neurological symptoms due to menigeosis neoplastica.
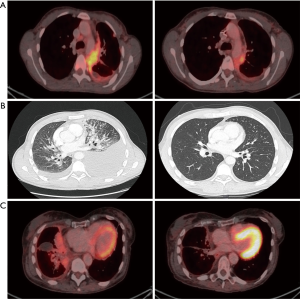
Case 2
A 38-year-old female patient presented with an adenocarcinoma of the lung with brain, bone and pleural metastases and an activating EGFR mutation (common Exon 19 deletion). Afatinib was started with 40 mg daily and had to be reduced to 30 mg daily due to grade 3 rush and diarrhea. Despite good initial treatment response the patient showed a clinically relevant tumor progression after 8 months, with hemorrhagic and malignant pleural effusion. Therefore the patient received thoracentesis regularly. Re-biopsy and next generation sequencing revealed the known Exon 19 deletion but no treatable resistance mutation. Accordingly, chemotherapy with cisplatin and pemetrexed was started. No further thoracentesis was needed and staging after the second cycle revealed partial remission (Figure 1B). After six cycles of chemotherapy maintenance treatment with pemetrexed was initiated but after a few weeks the patient’s performance status worsened and she developed progressive pleural carcinomatosis.
Case 3
A 64-year old-female patient was initially diagnosed with an adenocarcinoma of the lung with brain and pleural metastases and an activating EGFR mutation (common Exon 19 deletion). Treatment with afatinib was started (40 mg daily). Due to side effects the dosage had to be reduced to 30 mg daily. Following 15 months of afatinib treatment the patient developed peritoneal carcinomatosis and the rebiopsy revealed a T790M mutation. A third-line treatment with osimertinib was initiated. Six months later the patient was admitted to the hospital with progressive dyspnea caused by a hemorrhagic, malignant pleural effusion in combination with a massive pulmonary embolism and new skin metastases. Malignant cells of the pleural effusion harbored the known Exon 19 deletion. The skin metastases revealed an adenocarcinoma of the lung without any EGFR mutation. No T790M mutation was detectable at this timepoint. Accordingly, chemotherapy with cisplatin and pemetrexed was started although the patient was in bad general condition (ECOG 3). Following chemotherapy no further thoracentesis was needed, performance status improved and staging after two cycles showed partial remission (Figure 1C). After six cycles of chemotherapy a maintenance treatment with pemetrexed was performed, but also this patient developed a recurrent malignant pleural effusion after few weeks.
Discussion and short review of the literature
In this case series we describe three NSCLC patients with activating EGFR mutations. All of them showed a similar pattern of progression under TKI treatment: (I) no detectable resistance mutation; (II) pleural carcinomatosis with extensive hemorrhagic and malignant pleural effusion; and (III) very good response to chemotherapy with cisplatin and pemetrexed. As all three patients progressed after removal platinum from treatment schedule, platinum was presumably the more important part in this setting.
Pemetrexed was shown to prolong duration of response and progression free survival when added to TKI treatment in first line (6-8). Furthermore, some second line studies revealed good activity of pemetrexed after disease progression under first line TKI treatment in EGFR mutated NSCLC (9,10). However, Lee and colleagues showed that platinum based chemotherapy in combination with pemetrexed is more effective regarding response rate and progression free survival than pemetrexed alone in second line following first-line TKI treatment (11). Not only platinum but also other agents were evaluated for their effectiveness after resistance to TKI treatment. Bevacizumab in combination with pemetrexed was superior to the pemetrexed monotherapy as third-line therapy in patients with advanced EGFR-positive lung adenocarcinoma (12).
This small case series is in line with these results, indicating that chemotherapy with cisplatin and pemetrexed is a good treatment option for EGFR mutated patients progressing under TKI therapy without targetable resistance mutations and platinum is presumably the more important part in this setting. All pivotal studies of checkpoint inhibitors for second or third line revealed that EGFR mutated patients were the only subgroup that did not benefit from immunotherapy. Therefore, patients with EGFR mutations should receive chemotherapy after failure of TKI treatment. Even considering the risks and benefits of standard chemotherapeutic regimens it should also be discussed as a treatment approach for elderly patients or patients with poor performance. In line with this, a recent study showed similar response rates and PFS to platinum based salvage chemotherapy in elderly EGFR mutated patients after resistance of TKI treatment compared to younger patients (13).
Never the less, nearly all studies which investigated the effectiveness of chemotherapy after TKI resistance were conducted in Asian populations. This case study confirmed their results in three Caucasian patients. Furthermore, our patients received afatinib as first-line TKI treatment. Nowadays most international guidelines recommend third generations EGFR TKIs as first line therapy. This might change the therapeutic approach in such patients progressing under TKI treatment. Because this a small case series study, larger studies are needed to evaluate the most effective treatment and especially the best sequence of therapies in NSCLC patients with acquired resistance to TKI therapy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070-80. [Crossref] [PubMed]
- Zhong WZ, Zhou Q, Wu YL. The resistance mechanisms and treatment strategies for EGFR-mutant advanced non-small-cell lung cancer. Oncotarget 2017;8:71358-70. [Crossref] [PubMed]
- Cheng Y, Murakami H, Yang PC, et al. Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First-Line Therapy in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations. J Clin Oncol 2016;34:3258-66. [Crossref] [PubMed]
- Yang H, Deng Q, Qiu Y, et al. Erlotinib intercalating pemetrexed/cisplatin versus erlotinib alone in Chinese patients with brain metastases from lung adenocarcinoma: a prospective, non-randomised, concurrent controlled trial (NCT01578668). ESMO Open 2017;2:e000112. [Crossref] [PubMed]
- Yang JC, Mok T, Han B, et al. A Review of Regimens Combining Pemetrexed With an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in the Treatment of Advanced Nonsquamous Non-Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:27-34. [Crossref] [PubMed]
- Park S, Keam B, Kim SH, et al. Pemetrexed Singlet Versus Nonpemetrexed-Based Platinum Doublet as Second-Line Chemotherapy after First-Line Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor Failure in Non-small Cell Lung Cancer Patients with EGFR Mutations. Cancer Res Treat 2015;47:630-7. [Crossref] [PubMed]
- Li S, Zhou F, Ren S, et al. Response to pemetrexed rechallenge after acquired resistance of EGFR-TKI in a patient with advanced NSCLC. Lung Cancer 2014;84:203-5. [Crossref] [PubMed]
- Lee SJ, Sun JM, Lee SH, et al. Pemetrexed plus platinum versus pemetrexed alone in non-small cell lung cancer patients who have progressed after first-line EGFR TKIs. Lung Cancer 2015;90:261-6. [Crossref] [PubMed]
- Zhou CZ, Qin YY, Xie ZH, et al. Efficacy of third-line pemetrexed monotherapy versus pemetrexed combination with bevacizumab in patients with advanced EGFR mutation-positive lung adenocarcinoma. Chin J Cancer Res 2014;26:705-10. [PubMed]
- Tseng YH, Tseng YC, Lin YH, et al. Epidermal Growth Factor Receptor (EGFR)-Tyrosine Kinase Inhibitor Treatment and Salvage Chemotherapy in EGFR-Mutated Elderly Pulmonary Adenocarcinoma Patients. Oncologist 2015;20:758-66. [Crossref] [PubMed]
Cite this article as: Kauffmann-Guerrero D, Syunyaeva Z, Kahnert K, Tufman A. Excellent platinum dependent response to chemotherapy after relapse under TKI treatment in NSCLC with sensitizing EGFR mutations and no detectable resistance mutations: three case studies. AME Case Rep 2019;3:36.


