
Acute ischemic stroke as initial manifestation of undiagnosed iron deficiency anemia: case-report and literature review
Introduction
An acute cerebrovascular accident (CVA) is defined as a sudden onset of a neurological deficit caused by an abrupt focal injury to the central nervous system due to a disruption in the vascular supply (1). Approximately 80 percent of strokes are due to an ischemic cerebral infarction and 20 percent are due to a brain hemorrhage (2). Despite increased global awareness of the signs and symptoms of a stroke, it remains to be the second leading cause of death with someone suffering from a stroke every 40 seconds (3,4). Furthermore, strokes account for 1 out of 20 deaths in the United States with significant morbidities and mortalities leading to 34 billion US dollars in total hospital costs (2). At the same time, anemia is the most common blood disorder and has proven to be related to cardiovascular accidents (4). A recent epidemiological study estimated that 5.6% of the United States’ population met the criteria for the diagnosis of anemia. It is a global health problem that has a prevalence of 32.9% with iron deficiency anemia (IDA) being the most common cause worldwide (5,6). In about a quarter of ischemic strokes, the cause is undetermined. This may be due to the fact that a stroke workup is incomplete or delayed, multiple factors were at play causing a stroke, or that the stroke is genuinely undetermined after a full investigation, which will then be categorized as cryptogenic. IDA is an uncommon cause of acute ischemic stroke and has been reported in only three case reports (7-10). In this case, we present a 42-year-old female who developed an acute ischemic stroke that was concluded to be most likely secondary to IDA, after all other etiologies were ruled out. Our aim in this report is to identify IDA as a potential cause of ischemic strokes that could have been previously categorized as unknown etiology and thus, decrease the number of patients labeled to have cryptogenic strokes. We present the following article in accordance with the CARE reporting checklist (available at: http://dx.doi.org/10.21037/acr-20-72).
Case presentation
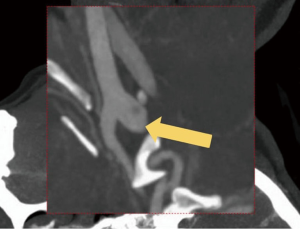
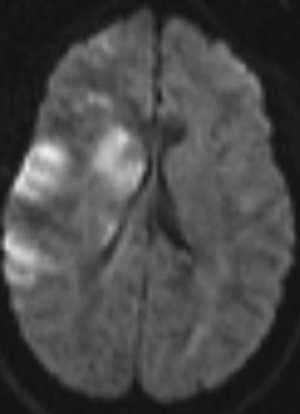
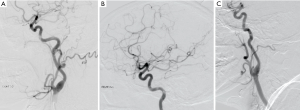
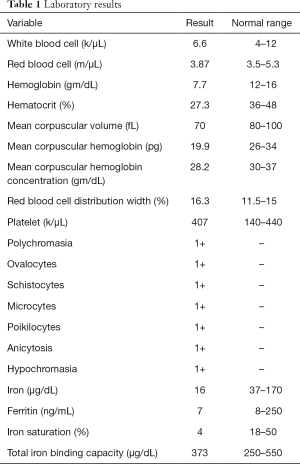
A right-handed 42-year-old previously healthy African American female presented to the emergency department following an unwitnessed fall. Subsequently, the patient was evaluated and resuscitated in the trauma bay per Advanced Trauma Life Support protocol as a Full Activation. No other prior medical and family history was known at presentation, and the patient denied taking either blood thinners or aspirin. However, upon further investigation, she has a family history significant for hypertension and diabetes. She reported occasional alcohol use and denied substance and tobacco use. On examination, she had a National Institute of Health Stroke Scale of 13, significant for left hemisensory loss, left upper and lower extremity drift, aphasia, and left facial palsy. She had a Glasgow Coma Score of 14 with altered mental status, and bilateral rightward eye deviation. Her blood pressure was found to be elevated (200/113 mmHg), which was controlled with intravenous boluses of labetalol and intravenous nicardipine infusion with a target systolic blood pressure of 160–180. Focused Assessment with Sonography in Trauma and chest radiograph of her chest and pelvis were all negative. Electrocardiogram revealed normal sinus rhythm, and the patient received a follow-up non-contrast computed tomography (CT) of her head, which revealed no abnormalities. She had a dislocation of her left second metacarpophalangeal joint from her fall, which was surgically repaired during her hospital stay. As she presented within the stroke reperfusion window, neurology was consulted, and the patient was given tissue plasminogen activator for a suspected right middle cerebral artery syndrome. Ensuing CT angiography (CTA) of the head and neck revealed no large vessel occlusion, as noted in Figure 1. Furthermore, no hemodynamic stenosis of the vasculature of the neck was noted. At 24-hours, her blood pressure normalized and she was started on an oral 81 mg aspirin tablet and 80 mg of atorvastatin. Following her CTA head and neck, the patient underwent a magnetic resonance imaging (MRI) of her brain, which revealed an infarct of her right middle cerebral artery and anterior cerebral artery territories (Figure 2). Further investigation with a transesophageal echocardiogram revealed a normal left ventricular ejection fraction of 65% and no thrombus nor emboli. Subsequent bubble test was negative for a patent foramen ovale. The patient then received an interventional cerebral angiogram of her bilateral common and internal carotid arteries. This revealed no evidence of any filling defects of her bilateral internal carotid arteries (Figure 3). Subsequently, a thorough hypercoagulable work up, including antinuclear antibodies, anticardiolipin, factor VIII, antithrombin, dilute Russell’s viper venom time, factor V, and Hex phospholipid neutralization, was unrevealing. Furthermore, the patient’s lipid panel revealed a high-density lipoprotein of 52 mg/dL and a low-density lipoprotein of 87 mg/dL. An iron study, blood smear, and complete blood cell count revealed a microcytic, hypochromic IDA, as depicted in Table 1. Upon further questioning, the patient reported a history of menorrhagia. She was started on oral doses of 325 mg ferrous sulfate every other day with improvement in her symptoms. The patient was discharged home on aspirin, atorvastatin, and ferrous sulfate. On her 4-week follow up, her cardiac monitor revealed no evidence of atrial fibrillation nor abnormal heart rhythm. 6-months later, her hemoglobin normalized to 13.2 g/dL and her iron replacement therapy was discontinued.
Full table
Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
With CVA being the second leading cause of death in the United States, strokes most commonly occur in individuals 65 years and older with ischemic type accounting for the majority (1-4,11). The primary mechanism behind developing a stroke is hypoperfusion of brain tissue and decreased nutrient supply, which occurs either through an arterial blockage or systemic hypoperfusion. The later mechanism primarily manifests when the body cannot sustain enough blood supply to maintain the brain’s demand. Theoretically, anything that would affect this mechanism can result in a stroke. In the Trial of Org 10172 in Acute Stroke Treatment classification, an undetermined cause for stroke can result from one out of three possible situations: an incomplete negative investigation, more than one possible cause, or the absence of a defined cause after an extensive investigation (12). It was rarely reported that chronic anemia could result in acute ischemic stroke, especially IDA, which might have been previously classified under undetermined or cryptogenic (7-10).
The prevalence of IDA in adolescent girls and women of childbearing age ranges between 2% and 5% (13). IDA usually presents in women of childbearing age due to menstruation, which results in a slow, chronic blood loss that allows the body to adapt to low concentrations of hemoglobin. Nevertheless, when blood loss occurs acutely, it can result in a rapid decline in the blood reservoir and lead to ischemia.
While many studies investigate the role IDA serves in CVA’s among the pediatric population, the relationship between the two has yet to be elucidated among the adult population. Several hypothesized pathophysiological mechanisms for IDA’s role in stroke include reactive thrombocytosis, hypercoagulable state, or inadequate oxygenation to the brain from the underlying anemia (14). Subedi et al. reported a case of a 4-year-old child who suffered an IDA attributed ischemic stroke, in which the child’s hemoglobin was 7.2 gm/dL on presentation (14). Munot et al. reported four cases of IDA attributed ischemic stroke, in which three of the four patients had venous sinus thrombosis (15). Also, Hartfield et al. reported a series of six children, who presented with an ischemic stroke or venous thrombosis after a viral prodrome, in which their findings support the evidence of a strong association between iron deficiency and ischemic events (16). Furthermore, a case-control study done in Egypt on 21 stroke cases showed that IDA was found in 57.1% of stroke cases with no identified cause, as compared to 26% of controls. They concluded that previously healthy children who developed a stroke are 3.8 times more likely to have IDA compared to healthy children who do not develop a stroke (95% CI: 1.3–11.2, P=0.005) (17). Moreover, in a case-control study conducted in Toronto, investigators examined 15 cases and 143 control subjects to assess the risk of strokes in patients with IDA. They discovered that IDA was significantly more common among case-patients, 8 of 15 (53%), than control subjects, 13 of 143 (9%), with a P value of <0.001. The significance of this study suggests that previously healthy children who suffer from strokes were 10 times more likely to have IDA than healthy children who do not suffer from a stroke (18).
Our case represents a middle-aged female who suffered an acute stroke while lacking major cardiovascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, or substance abuse. Although the patient’s blood pressure was initially elevated in the ED, all her subsequent blood pressure readings during her hospitalization were within the normal range and she did not require any blood pressure-lowering therapy. Therefore, with the absence of stroke risk factors, a normal initial laboratory stroke workup, and with the absence of atherosclerotic disease on imaging studies, this prompted us to further investigate the various causes of brain insults. The patient’s low hemoglobin and ongoing severe IDA proposed the possibility of an association. We conducted a systematic review of the literature for studies published from 1960 to October 2019 in PubMed, Scopus, Web of Science, and Cochrane Central databases. The following search terms were used: “Acute Ischemic Stroke” and “Iron Deficiency Anemia.” Our search was limited to individuals 18 years and older. Our search revealed a total of 8 patients (3 case-reports and 2 case series), as well as a population-based study. Our findings were similar to previous cases reported by Mehta et al. and Gopalratnam et al., who presented two cases of female patients aged 47 and 20-year-old respectively, who developed ischemic strokes in which common secondary causes were ruled-out. Their findings lead them to conclude an association between IDA and stroke secondary to hypoxia (7,8).
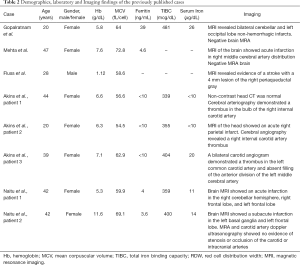
On the other hand, Fluss et al. and another case series reported a case of a 28-year-old male and three middle-aged female patients with IDA, who suffered from ischemic strokes and carotid artery thrombi, which was attributed to reactive thrombocytosis. This finding could explain the underlying process by which IDA can result in an ischemic stroke (9,19). Moreover, in 2014, two cases were reported in Japan concerning two female patients in their 40’s with IDA from chronic menorrhagia secondary to uterine fibroids who developed ischemic strokes. Their medical history was insignificant for any new neurological deficits occurring before the development of IDA or after the resolution of IDA, thus, suggesting the association between CVA secondary to IDA (10). Also, Chang et al. did a population base analysis in Taiwan which included 51,093 subjects. They found the adjusted odds ratio of ischemic stroke patients with prior IDA to be 1.45 (95% CI: 1.34–1.58) compared to stroke patient without IDA. Hence, they concluded a significant association between IDA and stroke (20). Further details of the previously mentioned cases’ demographics, laboratory, and imaging findings are listed in Table 2.
Full table
Limitation
As a case report we had Lack of ability to generalize, no possibility to establish cause-effect relationship, danger of over-interpretation, publication bias, retrospective design, and distraction of reader when focusing on the unusual.
Conclusions
We believe there is an association between IDA and ischemic stroke as seen in our report. While the disease’s pathophysiology remains unclear and warrants further studies, having iron studies being a part of a stroke work up, especially in young females, may prove to be beneficial. Further studies are needed to assess whether early treatment of IDA will have an impact on stroke prevalence. Our case report adds to the previous literature suggesting an association and we believe, with stronger evidence, that the proportion of ischemic strokes classified as cryptogenic will decrease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE guideline checklist. Available at: http://dx.doi.org/10.21037/acr-20-72
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-72). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89. [Crossref] [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 2014;129:e28-92. [Crossref] [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146-603. [Crossref] [PubMed]
- Yang Q, Tong X, Schieb L, et al. Vital signs: recent trends in stroke death rates—United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;66:933. [Crossref] [PubMed]
- Le CH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003-2012). PLoS One 2016;11:e0166635. [Crossref] [PubMed]
- Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615-24. [Crossref] [PubMed]
- Gopalratnam K, Woodson KA, Rangunwala J, et al. A Rare Case of Stroke Secondary to Iron Deficiency Anemia in a Young Female Patient. Case Rep Med 2017;2017:1684631. [Crossref] [PubMed]
- Mehta PJ, Chapman S, Jayam-Trouth A, et al. Acute ischemic stroke secondary to iron deficiency anemia: a case report. Case Rep Neurol Med. 2012;2012:487080. [Crossref] [PubMed]
- Fluss R, Zguri L, Rahme R, et al. Iron-deficiency Anemia Causes an Ischemic Stroke in a Young Man. Cureus 2019;11. [Crossref] [PubMed]
- Naito H, Naka H, Kanaya Y, et al. Two cases of acute ischemic stroke associated with iron deficiency anemia due to bleeding from uterine fibroids in middle-aged women. Intern Med 2014;53:2533-7. [Crossref] [PubMed]
- Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in US hospitals, 1989–2009. NCHS Data Brief 2012;18:23.
- Fonseca AC, Ferro JM. Cryptogenic stroke. Eur J Neurol 2015;22:618-23. [Crossref] [PubMed]
- Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA 1997;277:973-6. [Crossref] [PubMed]
- Subedi K, Koirala S, Basnet R, et al. Paediatric Stroke: A Rare Presentation of Iron Deficiency Anemia in a Four-Year-Old Child. Kathmandu Univ Med J 2016;14:282-384. (KUMJ). [PubMed]
- Munot P, De Vile C, Hemingway C, et al. Severe iron deficiency anaemia and ischaemic stroke in children. Arch Dis Child 2011;96:276-9. [Crossref] [PubMed]
- Hartfield DS, Lowry NJ, Keene DL, et al. Iron deficiency: a cause of stroke in infants and children. Pediatr Neurol 1997;16:50-3. [Crossref] [PubMed]
- Azab SF, Abdelsalam SM, Saleh SH, et al. Iron deficiency anemia as a risk factor for cerebrovascular events in early childhood: a case–control study. Ann Hematol 2014;93:571-6. [Crossref] [PubMed]
- Maguire JL. deveber G, Parkin PC. Association between iron-deficiency anemia and stroke in young children. Pediatrics 2007;120:1053-7. [Crossref] [PubMed]
- Akins PT, Glenn S, Nemeth PM, et al. Carotid artery thrombus associated with severe iron-deficiency anemia and thrombocytosis. Stroke 1996;27:1002-5. [Crossref] [PubMed]
- Chang YL, Hung SH, Ling W, et al. Association between ischemic stroke and iron-deficiency anemia: a population-based study. PLoS One 2013;8:e82952. [Crossref] [PubMed]
Cite this article as: Awad D, Kousa O, Essa A, Kuniyoshi J, Kousa H, Qasim A, Andukuri V, Kassim T. Acute ischemic stroke as initial manifestation of undiagnosed iron deficiency anemia: case-report and literature review. AME Case Rep 2020;4:23.


