
IgG4-related disease with multiple organs involvement was effectively controlled by glucocorticoids: a case report
Introduction
IgG4-related disease (IgG4-RD) is a chronic, progressive and autoimmune disease, which is usually characterized by infiltration of lymphocytes and IgG4+ plasma cells (>50% of infiltrated IgG4-positive cells) accompanied with fibrosis and sclerosis in tissues (1,2). IgG4-RD involves inflammation of multiple organs, such as eyes, tear ducts, salivary gland, thyroid gland, lung, kidney, biliary tract, pancreas, prostate, and testicles lymph nodes (3-5). Major clinical manifestations are related to the pancreas, bile duct, retroperitoneum, and salivary glands (6). Biliary cholangitis and autoimmune pancreatitis (AIP) are more common (7-9), and it has been recently discovered that Mikulicz’s disease (MD) is also related to IgG4-RD (10). Asymptomatic IgG4-RD, asymptomatic lymphadenopathy or mild submandibular gland enlargement can be observed without any need of treatment. However, special attention should be taken when some lesions are involved in the pancreas, biliary tree, aorta, mediastinum, kidneys, retroperitoneum and mesentery (11). A diagnosis of IgG4-RD requires a high index of clinical suspicion and is established by combining serum IgG4 concentration, other organ involvement, repeated imaging examinations, pathological examination, and response to glucocorticoids therapy (11,12). Now, there is no enough evidence to support the best choice of treatment for IgG4-RD, despite glucocorticoids are effective in nearly all patients with type 1 AIP (13,14).
Herein, we report that an elderly male patient who was diagnosed with IgG4-RD had multiple tissues and organs involvement, including biliary cholangitis, AIP, and MD, and was effectively treated with oral glucocorticoids. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-43) (15).
Case presentation
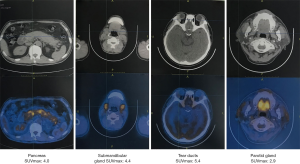
On July 5, 2018, a 53-year-old male was admitted to the Department of Hepatobiliary Surgery at our hospital due to intermittent abdominal pain spreading to the lower back for 2 months. He had suffered from bilateral mumps for more than 8 months. Magnetic resonance imaging (MRI) suggested diffuse thickening of the pancreas and dilatation of the pancreatic duct (Figure 1). Magnetic resonance cholangiopancreatography (MRCP) suggested that the intrahepatic bile duct was locally dilated, the diameter of the dilated bile duct was 1.2 cm, a beak-like stenosis at the end of the bile duct, and the proximal pancreatic duct was unclear (Figure 2). Computed tomography with positron emission tomography (CT-PET) indicated diffuse thickening of pancreas with increased metabolism, thickening of intrahepatic bile duct wall with increased metabolism, and enlargement of bilateral submandibular gland with increased metabolism (Figure 3). Laboratory tests showed that serum IgG concentration was 2,650 mg/dL (normal range: 700–1,600 mg/dL), serum carbohydrate antigen-199 (CA-199) concentration was 11.77 U/mL (normal range: 0.00–37.00 U/mL), serum amylase concentration was 468.00 U/L (normal range: 30.00–110.00 U/L), serum lipase concentration was 3,482.00 U/L (normal range: 23.00–300.00 U/L), serum alanine aminotransferase (ALT) was 62.00 U/L (normal range: 9.00–50.00 U/L), serum aspartate aminotransferase (AST) was 22.48 U/L (normal range: 15.00–40.00 U/L), serum total bilirubin (TBIL) was 6.40 µmol/L (normal range: 5.10–22.20 µmol/L), serum direct bilirubin (DBIL) was 3.30 µmol/L (normal range: 0.00–8.60 µmol/L), and r-glutamyl transpeptidase (r-GGT) was 348.04 U/L (normal range: 10.00–60.00 U/L) (Figure 4). Anti-nuclear antibody spectrum (ANA), anti-double stranded DNA antibody (ds-DNA), anti-extractable nuclear antibody (ENA), anti-cardiolipin antibody (ACA), rheumatoid factors and other autoantibodies were all negative. At the same time, hepatitis B virus and tuberculosis infection T cell detection were both negative. Then, he underwent the detection of serum IgG4 concentration at another hospital, which was 2,699 mg/dL (normal range: 3–201 mg/dL). Thus, his diagnosis was IgG4-RD including AIP, cholangitis with biliary tract obstruction, and MD.
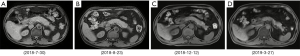
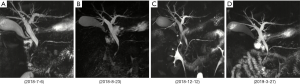
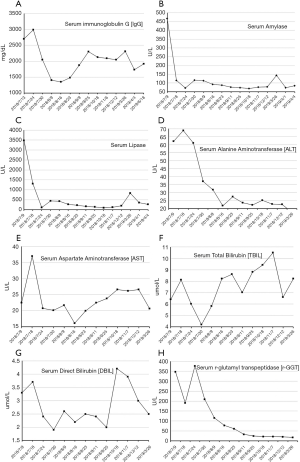
On July 19, 2018, the patient was transferred to our department. At that time, we initiated glucocorticoids treatment with prednisolone sodium succinate booster injection of 40 mg/day for 1 week. Then, prednisolone 32 mg/day was orally given for 1 week and was tapered to 24 mg/day for 1 week, finally the dosage of prednisolone was reduced to 16 mg/day for 3 weeks until he was discharged. On August 23, 2018, MRI (Figure 1B) and MRCP (Figure 2B) showed an improvement of biliary tract obstruction and AIP. Laboratory tests showed that serum IgG4 concentration was decreased to 1,050 mg/dL, serum IgG, serum amylase, serum lipase, serum ALT, serum AST, serum TBIL, serum DBIL, and r-GGT were reduced to normal.
The patient continued oral glucocorticoids with 4 mg/day after discharge. During regular follow-up, MRI (Figure 1C) and MRCP (Figure 2C) showed no significant change. Laboratory tests showed that serum IgG concentration ranged from 1,876 to 2,303 mg/dL, and serum amylase, serum lipase, serum ALT, serum AST, serum TBIL, serum DBIL, and r-GGT were normal. On December 12, 2018, MRI (Figure 1D) and MRCP (Figure 2D) were performed again and suggested no significant change.
On March 26, 2019, the patient was admitted to our department due to aggravated abdominal pain for 2 days. Laboratory tests showed that serum IgG concentration was 2,307 mg/dL, serum amylase concentration was 144 U/L, serum lipase concentration was 826 U/L, and serum ALT, AST, TBIL, DBIL, and r-GGT were normal. During this hospitalization, the patient was given oral glucocorticoids therapy with methylprednisolone 4 mg/day from March 26 to April 4, 2019. Then, serum IgG concentration was decreased to 1,732 mg/dL, serum amylase and lipase were reduced to normal. Considering the patient was in a good condition, he was discharged.
The patient continued oral glucocorticoids therapy after discharge, specifically 4 mg/day, and was followed up to July 8, 2019. The serum IgG concentration was re-examined on June 18, 2019 and the result was 1,917 mg/dL. Serum amylase, lipase, ALT, AST, TBIL, DBIL, and r-GGT levels were within the normal range. The patient was generally in a good condition and had no obvious complaints of discomfort.
Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
IgG4 concentration and IgG4-RD
In 2011, the Japanese IgG4 team proposed comprehensive diagnostic criteria for IgG4-RD with a major characteristic of serum IgG4 concentration >135 mg/dL (16). However, the diagnostic value of serum IgG4 concentration remains a bit controversial among the current literature. The sensitivity and negative predictive value of an elevated serum IgG4 concentration for the diagnosis of IgG4-RD are high (17). A retrospective analysis by Carruthers et al. also suggested that a higher serum IgG4 concentration had a higher likelihood of diagnosing with IgG4-RD (18). Among 380 patients analyzed, 72 patients had either probable or definite IgG4-RD and 308 patients had no IgG4-RD. Sixty-five of the 72 IgG4-RD patients had a higher serum IgG4 concentration (mean: 405 mg/dL; range: 140–2,000 mg/dL), indicating a high sensitivity of 90%. However, 125 of 308 patients without IgG4-RD also had a higher serum IgG4 concentration (mean: 234 mg/dL; range: 135–1,180 mg/dL), indicating a low specificity of 60%. Indeed, it is essential to notice that an elevated IgG4 concentration can be found in multiple conditions unrelated to IgG4-RD, such as autoimmune conditions, asthma, allergies, multicentric Castleman disease, plasma cell dyscrasia, and malignancies (4). Our patient’s serum IgG4 concentration was significantly increased to 2,699 mg/dL at the time of his first diagnosis and decreased to 1,050 mg/dL after glucocorticoids treatment during the first hospitalization.
Multiple organ involvement in IgG4-RD
IgG4-RD is now well known to affect a wide range of organs with a chronic fibro-inflammatory condition (19) in the pancreas (AIP) and biliary systems (cholangitis with biliary tract obstruction) as well as salivary and lacrimal glands (MD) (3,20,21). An intuitive approach to classify IgG4-RD on the basis of clinicopathological characteristics is to differentiate two overlapping subtypes: proliferative and fibrotic. Patients with proliferative IgG4-RD tend to have high serum concentrations of IgG4, a high probability of multi-organ diseases, and a better response to treatment (22,23). Our patient had multiple organs involvement presenting with increased metabolism on CT-PET and had diffuse thickening of the pancreas, dilatation of the pancreatic duct, and cholangitis with biliary obstruction radiologically. Since all the organs involved were glands, our patient might be clinically classified as proliferative subtype.
Treatment
Glucocorticoids are the first-line treatment for remission induction in all patients with active and untreated IgG4-RD, and the treatment should be individualized for each patient (5). On the other hand, the response to glucocorticoids has become a part of the diagnostic criteria of AIP (11). For patients with high serum IgG4 concentration and multi-organ diseases, remissions are unlikely to be induced if glucocorticoid courses were not lengthy enough (24). Okazaki and colleagues (25) recommended that initial oral prednisolone dose for induction of remission was 0.6 mg/kg/day, which was administered for 2–4 weeks. The dose was then tapered by 5 mg every 1–2 weeks according to the changes in clinical manifestations, laboratory tests, such as liver transaminase, IgG or IgG4 concentration, and repeated imaging findings, such as US, CT, MRCP, and ERCP. Finally, the dose was tapered to a maintenance dose (2.5–5 mg/day) over a period of 2–3 months, and the termination of maintenance therapy should be planned within 3 years in cases with radiological and serological improvement. Our patient insisted on oral glucocorticoids with 4 mg/day after discharge. Serum IgG concentration was decreased, and imaging examination suggested a slight improvement of sclerosing cholangitis and AIP. IgG4-RD is prone to recurrence, the risk of which increases with the number of organs affected at baseline and baseline serum IgG4 concentration (24). Unfortunately, our patient relapsed 8 months after the first discharge with high serum IgG4 concentration (2,650 mg/dL) and multiple organs involved and was re-admitted for oral glucocorticoids therapy. Indeed, a retrospective study reported a relapse in 40% (38/96) of AIP patients who underwent maintenance therapy with low-dose glucocorticoids. Notably, 26% (10/38) of the relapsers had a maintenance therapy with a dose of prednisolone >5 mg/day (24). In another study, 54% (14/26) of patients who discontinued maintenance prednisolone experienced relapses (26). At present, our patient is well controlled by glucocorticoids, and no additional treatment is given.
Conclusions
The patient could be diagnosed with IgG4-RD based on laboratory tests, repeated imaging examination, and effective glucocorticoids treatment, despite the biopsy was missing. His clinical symptoms were in remission, but imaging examination and serum IgG4 concentration remission were not significant. However, the dose was not further increased after discharge, because the patient was worried about the side effects of glucocorticoids. We are continuing to follow up this patient for his dynamic change in clinical presentations, serum IgG4 concentration, imaging examinations, and potential side effects secondary to oral glucocorticoids.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-43
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okazaki K, Uchida K, Koyabu M, et al. IgG4 cholangiopathy: current concept, diagnosis, and pathogenesis. J Hepatol 2014;61:690-5. [Crossref] [PubMed]
- Okazaki K, Uchida K. Current perspectives on autoimmune pancreatitis and IgG4-related disease. Proc Jpn Acad Ser B Phys Biol Sci 2018;94:412-27. [Crossref] [PubMed]
- Yamamoto M, Takahashi H, Ohara M, et al. A new conceptualization for Mikulicz’s disease as an IgG4-related plasmacytic disease. Mod Rheumatol 2006;16:335-40. [Crossref] [PubMed]
- Abraham M, Khosroshahi A. Diagnostic and treatment workup for IgG4-related disease. Expert Rev Clin Immunol 2017;13:867-75. [Crossref] [PubMed]
- Stone JH. IgG4-related disease: nomenclature, clinical features, and treatment. Semin Diagn Pathol 2012;29:177-90. [Crossref] [PubMed]
- Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol 2006;41:613-25. [Crossref] [PubMed]
- Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology 2008;134:706-15. [Crossref] [PubMed]
- Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic’s HISORt criteria. J Gastroenterol 2007;42 Suppl 18:39-41. [Crossref] [PubMed]
- Hubers LM, Maillette de Buy Wenniger LJ, Doorenspleet ME, et al. IgG4-associated cholangitis: a comprehensive review. Clin Rev Allergy Immunol 2015;48:198-206. [Crossref] [PubMed]
- Himi T, Takano K, Yamamoto M, et al. A novel concept of Mikulicz’s disease as IgG4-related disease. Auris Nasus Larynx 2012;39:9-17. [Crossref] [PubMed]
- Khosroshahi A, Wallace ZS, Crowe JL, et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol 2015;67:1688-99. [Crossref] [PubMed]
- El Euch M, Hddad S, Mahfoudhi M, et al. A Case of Type 1 Autoimmune Pancreatitis (AIP), a Form of IgG4-Related Disease (IgG4-RD). Am J Case Rep 2017;18:822-5. [Crossref] [PubMed]
- Okazaki K, Chari ST, Frulloni L, et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology 2017;17:1-6. [Crossref] [PubMed]
- Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013;62:1771-6. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Umehara H, Okazaki K, Kawano M, et al. How to diagnose IgG4-related disease. Ann Rheum Dis 2017;76:e46. [Crossref] [PubMed]
- Boonstra K, Culver EL, de Buy Wenniger LM, et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 2014;59:1954-63. [Crossref] [PubMed]
- Carruthers MN, Khosroshahi A, Augustin T, et al. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14-8. [Crossref] [PubMed]
- Stone JH, Brito-Zeron P, Bosch X, et al. Diagnostic Approach to the Complexity of IgG4-Related Disease. Mayo Clin Proc 2015;90:927-39. [Crossref] [PubMed]
- de Pretis N, Amodio A, Frulloni L. Updates in the field of autoimmune pancreatitis: a clinical guide. Expert Rev Gastroenterol Hepatol 2018;12:705-9. [Crossref] [PubMed]
- Joshi D, Webster GJ. Biliary and hepatic involvement in IgG4-related disease. Aliment Pharmacol Ther 2014;40:1251-61. [Crossref] [PubMed]
- Wang M, Zhang P, Lin W, et al. Differences and similarities between IgG4-related disease with and without dacryoadenitis and sialoadenitis: clinical manifestations and treatment efficacy. Arthritis Res Ther 2019;21:44. [Crossref] [PubMed]
- Cortazar FB, Stone JH. IgG4-related disease and the kidney. Nat Rev Nephrol 2015;11:599-609. [Crossref] [PubMed]
- Zhang W, Stone JH. Management of IgG4-related disease. Lancet Rheumatol 2019;1:e55-65. [Crossref]
- Okazaki K, Kawa S, Kamisawa T, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol 2014;49:567-88. [Crossref] [PubMed]
- Raina A, Yadav D, Krasinskas AM, et al. Evaluation and Management of Autoimmune Pancreatitis: Experience at a Large US Center. Am J Gastroenterol 2009;104:2295-306. [Crossref] [PubMed]
Cite this article as: Wang S, Xu X, Bai Z, Yi F, Wang R, Guo X, Qi X. IgG4-related disease with multiple organs involvement was effectively controlled by glucocorticoids: a case report. AME Case Rep 2020;4:22.


