
Two case reports of rare diseases occurring in rare parts: splenic vein solitary fibrous tumor and liver solitary fibrous tumor
Introduction
Solitary fibrous tumor (SFT) is an uncommon mesenchymal neoplasm that is characterized by a patternless histological architecture, an intrachromosomal fusion gene NAB2-STAT6 in chromosome 12, and nuclear immunoreactivity for signal transducer and activator of transcription 6 (STAT6) (1). This tumor was first described by Klemperer and Rabin in 1931 (2). In 2013, the WHO classified SFT as a tumor derived from fibroblasts or myofibroblasts, which rarely metastasize (3). Most SFTs are benign lesions, and the prognosis is well after surgical resection. However, there are still some malignant lesions which can lead to the surrounding tissue infiltration, local recurrence, and distant metastasis (4). Surgical resection is still the core of its treatment. The reports about SFT were mainly concentrated in the pleura, besides some reports suggested that it could occur in any locations, including meninges, thyroid (5), round ligament of the liver (6), pancreas (4), and the liver SFT was first reported in 1959 (7). But so far there have been no reports of splenic veins SFT, neither reports of liver recurrence SFT cured by orthotopic liver transplantation (OLT).
We present the following cases in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-142).
Case presentation
Case 1
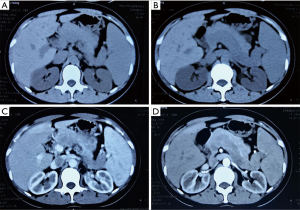
A 37-year-old female patient was admitted to the hospital because of abdominal pain for one week. Physical examination upon admission revealed splenomegaly. Laboratory tests showed that, (WBC) count was 2.70×109/L (normal range, 4×109–10×109/L), platelet count was 88×109/L (normal range, 100×109–300×109/L), and red blood cell (RBC) count was 3.61×1012/L (normal range, 3.8×109–5.1×1012/L); liver function and blood coagulation test was normal, and tumor markers were negative. Computed tomography (CT) scan indicated splenomegaly, splenic vein dilatation, low-density filling defect, no obvious enhancement in arterial or portal phase (Figure 1). The splenic vein tumor and spleen resection were performed in 2009. At surgery, the tumor was yellow-white debris, and the spleen was enlarged (16.0 cm × 5.0 cm), the splenic vein was tortuous and dilated. Histologically, the tumor was principally composed of spindle cells. Immunohistochemically, the tumor cells were positive for vimentin, CD34, and Bcl-2 and negative for smooth muscle actin (SMA), s-100, CD99, and CD117. And we also found Ki-67 (+) >2%. Therefore, the tumor was diagnosed to be a malignant SFT (MSFT) and the patient was discharged on the 9th day after the operation.
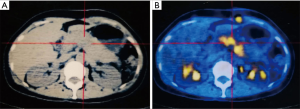
A month after the operation, CT and positron emission tomography/computed tomography (PET/CT) scan found SFT recurrence, and there was an 8.0 cm × 2.5 cm low-density filling defect in the splenic vein, and no signs of tumor and metastasis were found in other sites (Figure 2). Although the pancreas was not invaded by the tumor, considering the cavernous transformation of splenic vein secondary to tumor embolus, the formation of collateral circulation and adhesion among the splenic vein, the body and tail of pancreatic, we surgically resected the tumor again and resected the body and tail of pancreas and regional lymph nodes to ensure the R0 resection during the operation. Histology and immunohistochemistry test results were the same as before, but this time we found that the tumor broke through the blood vessel wall and infiltrated the surrounding tissues. The patient was discharged two weeks later without any complications, and was followed up every year for 4 years during which she did not receive any radiotherapy, chemotherapy, or molecular targeted therapy.
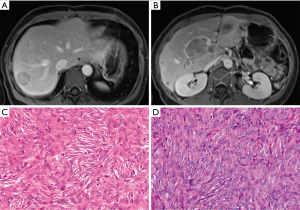
Unfortunately, 49 months later (in July 2013), magnetic resonance imaging (MRI) revealed two metastatic SFTs in the liver and one tumor in the portal vein, measuring 0.3 cm, 3.0 cm, 8.1 cm × 4.8 cm × 3.9 cm, respectively (Figure 3A,B). Partial liver and portal vein tumor resections were performed. Pathological results confirmed hepatic metastatic MSFT (Figure 3C,D). Immunohistochemistry showed the tumor cells were positive for vimentin, CD34, Bcl-2, epithelial membrane antigen (EMA), and negative for SMA, s-100, STAT-6, CD99, CD117, and Ki-67 (about 5%). However, 39 months later (in October 2016), the MRI scan found a local recurrence of the right posterior lobe of the liver and portal vein again, and surgical resection was performed, the pathological examination result was the same as before (Figure 3C,D). The patient’s liver function was normal and there were no obvious complications after these two surgical operations, and she was discharged from the hospital on the 9th and 8th days after surgery, respectively.
At present, the patient is still being followed up in our hospital and is in generally good condition, with no symptoms of fatigue, weight loss or abdominal pain, etc.
Case 2
A 54-year-old male was admitted to the hospital because of routine medical checkups, with no uncomfortable symptoms (in March 2008). Abdominal ultrasonography revealed a 5 cm × 6 cm heterogeneous hypoechoic mass in the right lobe of the liver, without portal vein, hepatic vein, or Inferior vena cava involvement. PET-CT scan revealed multiple neoplastic lesions in the liver, spleen, and left side of the chest wall, high probability of malignancy. The surgeons decided to perform two operations to remove these tumors. In April 2008, left chest wall tumor resection was performed, and in May 2008, partial liver (S4/S6) and partial spleen resection was performed, pathological results confirmed SFT of the chest wall, liver, and spleen.
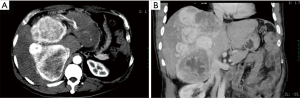
Seventy-nine months later (in September 2014), he was admitted to our hospital again because of a mild “heavy” feeling in the abdomen. CT and PET/CT scan demonstrated that the liver had a large solid mass with multiple leaves and rich blood supply, measuring 23 cm × 18 cm × 11 cm (Figure 4A,B). Liver biopsy confirmed the recurrence of SFT. And the laboratory data indicated that liver function was impaired by SFT, with ALT level at 186 U/L (normal range, 5–35 U/L) and TBIL level at 53 mmol/L (normal range, 3–20 mmol/L). The preoperative evaluation showed that SFT resection may result in insufficient residual liver volume. Therefore, we performed LT on December 5, 2014 (Figure 5). We separated the abdominal adhesions and explored the abdominal cavity, and there was no tumor recurrence in the original surgical area or abdominal metastasis, and we only found multiple masses in the liver. The graft, which weighed 1,125 g, was from a 45-year-old, ABO compatible donor, who donated after brain death (DBD). Standard OLT (SOLT) was performed after graft trimming. The superior and inferior vena cava of the donor and recipient were anastomosed with Prolene 3-0 and 4-0, the portal vein with Prolene 5-0, the common hepatic artery with Prolene 7-0, and the bile ducts with polydioxanone suture (PDS) 6-0. The operation went well and the patient was in good condition. On the first day after LT, the tacrolimus was taken orally, and its blood concentration was maintained at 8–12 ng/mL at the 1st month, 7–10 ng/mL at the 2nd–6th month, and 5–8 ng/mL after the 6th month. No obvious complications occurred after LT, and the patient was discharged at the second week after surgery. Histologically, this tumor was composed of spindle cells with hyper-cellular areas and hypo-cellular areas (Figure 6A). In hyper-cellular areas there were significant nuclear pleomorphisms and mitotic figures [more than 4 per 10 high-power fields (HPFs)]. In other areas many collagen fibers could be observed, and the tumor cells were observed to infiltrate adjacent tissues. Immunohistochemically, the tumor cells were positive for vimentin, CD34, CD99, and Bcl-2, but no immunoreactivity was noted with staining for Syn, chromogranin A (Cg A), anaplastic lymphoma kinase (ALK), SMA, s100 and CD117. Additionally, focal tumor cells were sporadically positive for CD56, and Ki-67 (+) (5% to 10%) (Figure 6B,C,D).
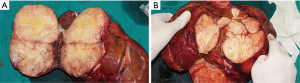

No tumor recurred in this patient during 6 years’ follow-up, and importantly, he is also in a good condition right now.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Clinical characteristics
SFTs grow slowly, and symptoms are often related to the size or site of the tumor, Symptoms that had been reported in other cases include dyspnea, fatigue, abdominal pain, nausea, vomiting, weight loss and hypoglycemia (2,8-11). Nausea and vomiting were related to the compression from the tumor on the esophagus and stomach, and hypoglycemia may be associated with IGF-2, while the reason of fatigue has not been clearly explained yet. Besides, many case reports showed that the disease is asymptomatic. In our case report, the first patient complained abdominal pain; the other one showed no symptoms at first, and later complained a mild “heavy” feeling in the abdomen at the second hospital admission.
Currently, there is no relevant report on primary splenic vein SFT and only one case of pancreas SFT invading splenic vein has been reported (4), therefore, other clinical characteristics of splenic vein SFT are currently unknown. But since Nevius and Friedman first described about liver SFT in 1959 (7), there have been 96 case reports of liver SFT in English literatures: liver SFTs seem to be more likely to occur in female patients, with a female: male predominance of approximately 1.37:1, The mean age was 56.3 years old, The tumor can be found in either the right or the left hepatic lobe and the mean tumor size was 16.3 (2.0–35.0) cm (Table 1).

Full table
Diagnosis
The characteristic of imaging examinations is not typical; however, CT and MRI can detect the location, size, and relationship with surrounding tissues of the SFTs, and reflect the internal histology of the tumor. PET/CT can analyze SFTs in both functional metabolism and anatomical location, simultaneously; and can be used to evaluate whether there is a distant metastasis before surgery.
SFTs were usually confirmed by immunohistochemistry, through tissue biopsy or resected specimens. Meanwhile, CD34 expression was the most consistent finding reported to date, present 90–95%, other markers include vimentin, CD99, EMA, and Bcl-2 (2,12). In these two patients, vimentin, CD34, and Bcl-2 were all positive, and only the second case was CD99 positive. However, all these markers may also be positive in other soft tissue tumors (13). The latest findings suggest that STAT6 is a highly sensitive and almost completely specific immunohistochemical marker for SFT, which is beneficial for differentiating SFT from other tumors (12,14). Doyle et al. evaluated whole-tissue sections of 231 tumors, including SFTs and other benign and malignant mesenchymal neoplasms and sarcomatoid mesothelioma, 98% of SFTs showed nuclear expression of STAT6, while STAT6 expression was negative for all other tumor types (12). The immunohistochemistry of case 1 in this paper did not show positive STAT6, which may be related to the detection method. The positive rate of CD34 in Table 1 is similar to the above-mentioned data, while the low positive rate of STAT6 was mainly because that the test was not arranged (Table 1).
Treatments
Currently, surgical resection remains the first option of treatment for SFT, and the overall 10-year survival rate in surgical studies with clear margins ranged from 54% and 89% (15,16), and to achieve clear margins, it is necessary to expand the resection range. Although the first MSFT patient underwent 4 surgical resections, she is still in good condition. In addition, some new surgical methods have been reported. Sun et al. used liver auto-transplantation to treat a large liver SFT patient (17); Zhu et al. used endoscopic submucosal dissection to treat a rare esophageal SFT patient (18), and El-Khouli et al. used TACE to treat unresectable liver SFT patient (19). For the recurrent SFT in our second patient, we adopted OLT, and in the 6 years of follow-up, he was also in good condition with no tumor recurrence and metastasis.
In terms of adjuvant therapy, Bishop et al. and Salas et al. suggested that surgery combined with radiotherapy could achieve better effect than surgery alone (20,21), however, the sample size was small and larger sample studies are needed to confirm this opinion. Stacchiotti et al reported that 8 patients with SFTs were treated with dacarbazine monotherapy and 12 patients were treated with doxorubicin/dacarbazine combination, and doxorubicin/dacarbazine combination caused a max tumor volume inhibition >80%, the median progression-free survival (PFS) was longer (22). Martin-Broto et al. conducted the first phase 2 trial of pazopanib for SFTs and 58% of the patients with typical SFT had a partial response to pazopanib, which suggested that pazopanib has activity in the treatment of SFTs (1). Considering the financial situation of two patients, genetic testing and follow-up adjuvant therapy were not conducted, meanwhile, appropriate and effective adjuvant therapy was still being explored.
Prognosis
The nature of tumor, namely, whether it is benign or malignant, affects the prognosis of patients. Most SFTs reported in the literature are benign (>80%) (4). For the liver SFT, MSFT accounts for 22.9%, and there is no difference between males and females (Table 2).

Full table
Tumors can be considered to be malignant if they have the following characteristics: infiltrative margins, high cellularity, prominent cellular atypia, tumor necrosis and increased mitotic activity (>4 mitoses per 10 HPF) (3), Beltrán et al. found that Ki-67 higher than 5% could serve as a marker of malignancy, and Ki-67 positivity in 5% or less as a marker of benign (9). A long-term follow-up showed that increasing mitotic count correlates with increasing probability of metastases and shortening overall survival (23). The short-term recurrence of case 2 may be related to mitotic count. Meanwhile, SFT patients with tumors larger than 10 cm have a poor prognosis (24). However, there is one reported case of a malignant SFT measuring only 3 cm in diameter (Table 2). Besides, Martin-Broto et al. suggested that overexpression of CD209 was related to poor prognosis in SFTs (1).
Conclusions
We report two cases of splenic vein MSFT with hypersplenism and recurrent liver SFT treated by OLT for the first time. Surgical resection is an important treatment approach; radiotherapy, chemotherapy and targeted therapy are used to control local recurrence and may be effective for distant metastasis, further research is needed to confirm it, liver transplantation received in the second case may be a new option for the huge liver SFT.
In summary, since splenic vein SFT and other vascular system-related SFT, and liver SFT are very rare, these case reports are of importance for further understanding of this disease and improvement of its treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist for case reports. Available at http://dx.doi.org/10.21037/acr-20-142
Peer Review File: Available at http://dx.doi.org/10.21037/acr-20-142
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-142). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martin-Broto J, Cruz J, Penel N, et al. Pazopanib for treatment of typical solitary fibrous tumours: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:456-66. [Crossref] [PubMed]
- Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med 1992;22:1-31. [Crossref] [PubMed]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95-104. [Crossref] [PubMed]
- Li J, Li J, Xiong Y, et al. Atypical/malignant solitary fibrous tumor of the pancreas with spleen vein invasion: Case report and literature review. Medicine (Baltimore) 2020;99:e19783 [Crossref] [PubMed]
- Thompson LDR, Wei C, Rooper LM, et al. Thyroid Gland Solitary Fibrous Tumor: Report of 3 Cases and a Comprehensive Review of the Literature. Head Neck Pathol 2019;13:597-605. [Crossref] [PubMed]
- Beyer L, Delpero JR, Chetaille B, et al. Solitary fibrous tumor in the round ligament of the liver: a fortunate intraoperative discovery. Case Rep Oncol 2012;5:187-94. [Crossref] [PubMed]
- Nevius DB, Friedman NB. Mesotheliomas and extraovarian thecomas with hypoglycemic and nephrotic syndromes. Cancer 1959;12:1263-9. [Crossref] [PubMed]
- Fama F, Le Bouc Y, Barrande G, et al. Solitary fibrous tumour of the liver with IGF-II-related hypoglycaemia. A case report. Langenbecks Arch Surg 2008;393:611-6. [Crossref] [PubMed]
- Beltrán MA. Solitary Fibrous Tumor of the Liver: a Review of the Current Knowledge and Report of a New Case. J Gastrointest Cancer 2015;46:333-42. [Crossref] [PubMed]
- Kim H, Damjanov I. Localized fibrous mesothelioma of the liver. Report of a giant tumor studied by light and electron microscopy. Cancer 1983;52:1662-5. [Crossref] [PubMed]
- Delvecchio A, Duda L, Conticchio M, et al. Doege-Potter syndrome by malignant solitary fibrous tumor of the liver: A case report and review of literature. World J Gastrointest Surg 2019;11:348-57. [Crossref] [PubMed]
- Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-5. [Crossref] [PubMed]
- Suster S, Fisher C, Moran CA. Expression of bcl-2 oncoprotein in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces, and gastrointestinal tract. Am J Surg Pathol 1998;22:863-72. [Crossref] [PubMed]
- Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180-5. [Crossref] [PubMed]
- Spitz FR, Bouvet M, Pisters PW, et al. Hemangiopericytoma: a 20-year single-institution experience. Ann Surg Oncol 1998;5:350-5. [Crossref] [PubMed]
- Espat NJ, Lewis JJ, Leung D, et al. Conventional hemangiopericytoma: modern analysis of outcome. Cancer 2002;95:1746-51. [Crossref] [PubMed]
- Sun Z, Ding Y, Jiang Y, et al. Ex situ hepatectomy and liver autotransplantation for a treating giant solitary fibrous tumor: A case report. Oncol Lett 2019;17:1042-52. [PubMed]
- Zhu XS, Dai YC, Chen ZX. Giant Solitary Fibrous Tumor of Esophagus Resected by Endoscopic Submucosal Dissection. Ann Thorac Surg 2015;100:2340-3. [Crossref] [PubMed]
- El-Khouli RH, Geschwind JF, Bluemke DA, et al. Solitary fibrous tumor of the liver: magnetic resonance imaging evaluation and treatment with transarterial chemoembolization. J Comput Assist Tomogr 2008;32:769-71. [Crossref] [PubMed]
- Bishop AJ, Zagars GK, Demicco EG, et al. Soft Tissue Solitary Fibrous Tumor: Combined Surgery and Radiation Therapy Results in Excellent Local Control. Am J Clin Oncol 2018;41:81-5. [Crossref] [PubMed]
- Salas S, Resseguier N, Blay JY, et al. Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann Oncol 2017;28:1979-87. [Crossref] [PubMed]
- Stacchiotti S, Saponara M, Frapolli R, et al. Patient-derived solitary fibrous tumour xenografts predict high sensitivity to doxorubicin/dacarbazine combination confirmed in the clinic and highlight the potential effectiveness of trabectedin or eribulin against this tumour. Eur J Cancer 2017;76:84-92. [Crossref] [PubMed]
- O'Neill AC, Tirumani SH, Do WS, et al. Metastatic Patterns of Solitary Fibrous Tumors: A Single-Institution Experience. AJR Am J Roentgenol 2017;208:2-9. [Crossref] [PubMed]
- Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002;94:1057-68. [Crossref] [PubMed]
Cite this article as: Wang W, Bao B, Hu A, Zhu X, Chen Q. Two case reports of rare diseases occurring in rare parts: splenic vein solitary fibrous tumor and liver solitary fibrous tumor. AME Case Rep 2021;5:17.


