
Recurrent infantile digital fibromatosis with HPV infection: a case report
Introduction
Infantile digital fibromatosis (IDF), also called inclusion body fibromatosis (IBF), is one of rare, non-malignant pediatric myofibroblastic tumors, which is often limited to fingers and toes (1). It is traditionally characterized by round eosinophilic inclusion bodies of different sizes, similar to erythrocyte in the parakarytoplasm (2). Human papillomavirus (HPV) is a circular icosahedral double-stranded DNA virus (3). It most often infects human skin and mucous epithelium. HPV, with recurrent IDF, has not been mentioned in the previous literature. Here, we report a case of recurrent IDF with HPV infection.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-95).
Case presentation
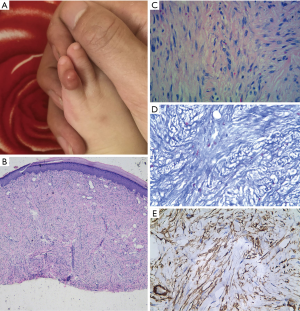
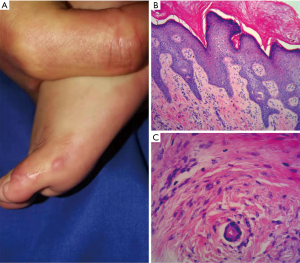
A 9-month-old female baby presented to our dermatologic clinic with a progressive enlarged, hemispheric and dull-red mass on the dorsum of left little toe since 3 months of age (Figure 1A). No traumatic or inflammatory history was reported and systemic examinations were normal. To differentiate benign from malignant nature of the tumor and make clear the diagnosis, it was examined by biopsy with patient's informed consent in July 2018. The histological finding is that neoplasm mainly located in the dermis and intradermis with obscure demarcation (Figure 1B). The intracytoplasmic eosinophilic inclusion bodies were distributed unevenly without refraction by HEstaining (Figure 1C), showed flesh-red with Masson-trichrome staining (Figure 1D) and SMA positively with immunohistochemistry (Figure 1E). The symbolic histological characteristics of intracytoplasmic eosinophilic inclusion bodies contributed to the diagnosis of IDF. Subsequently, the remaining tumor tissues were removed by surgical excision completely. However, it recurred after 6 months (Figure 2A), and then the tumor was treated with complete excision again with patient's informed consent in January 2019. Histopathological examination also indicated hyperkeratosis, acanthosis, papillomatosis, and characteristic koilocytes (Figure 2B) besides eosinophilic inclusion bodies (Figure 2C). The feature suggested the possibility of HPV infection. Viral metagenomics revealed the HPV-4 genomes in the tumor tissue. By tracing the familial history carefully, the patient's grandmother was found to be confirmed as verruca plana 4 months before, but others have no history of similar diseases or HPV infections. In April 2019, the tumor recurred again and the case is still being followed up. For a summary timeline events, please see Figure 3. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from baby’s father.
Discussion
IDF is a rare fibromatosis, presenting as the proliferation of myofibroblasts or fibroblasts in the dermis (4). The clinical presentation is very distinctive with firm, non‐tender, hemispheric and dull-red nodules. Nodules are more prevalent on the dorsal of digits of infants, especially second toe, and can also be seen in other parts, such as thigh, forearm, breast and scrotum, or without digits involvement (5). About 86% cases present with nodules within the first year of life. These nodules usually experience a period of slow growth and rapid growth, and eventually reach a steady-state or regress (6).
In order to make a clear diagnosis, three potential dermoscopic features of IDF are white dots, linear white constructions and telangiectasia, which were once described by Tomii in detail (7). But the histological feature is characteristic, with paranuclear eosinophilic inclusion bodies, which is the most valuable pathological manifestation. Although IDF is a benign disease with spontaneous regression tendency, the recurrence rate post incomplete excision can still reach 75% (5). Agarwal et al. (1) recommended that cytological diagnosis should be made to avoid unnecessary surgery and postoperative complications. The fine needle aspiration cytology (FNAC) as a minimally invasive procedure is adopted more and more widely in suggesting probable preoperative nature, especially for pediatric benign fibroblastic or myofibroblastic tumors. The main finding in IDF by FNAC smears is that plump oval to spindle shaped cells embed in a collagenous stroma with round to oval nuclei and light-stained cytoplasm, but without mitosis or necrosis (8).
Complete surgical excision is still the major therapeutic way if the tumors become larger, produce pains or affect normal structures or functions. Mohs micrographic surgery (MMS) is helpful to confirm the surgical margin and reduce recurrence (9). Local skin flaps coverage, split-thickness skin autograft or reepithelization based on wide surgical excision can provide an effective solution for recurrent IDF (10). In addition to this, nonsurgical approaches, such as topical or intralesional steroids and intralesional 5-fluorouracil may play an anti-fibrosis effect to shorten the time of spontaneous regression (11). It has been reported that cryotherapy combined with postoperative functional exercise achieved a satisfactory result for IDF with joint contracture and severe functional defects (12). For asymptomatic lesions, observing for spontaneous regression could be considered in clinical practice.
The pathogenesis of IDF remains elusive up to the present. Reye (4) speculated that virus was the reason of IDF, because the inclusion bodies were pyroninophilic and feulgen negative, and they all indicated the possibility of RNA. However, Zhu et al. (13) failed to detect HPV and HSV DNA in IDF by PCR, and suggested that IDF had nothing to do with viral infection. Afterwards, it was proved that the inclusion bodies are densely packed vimentin and the main cause is possibly related to the abnormal aggregation of myofilament (2). And no sign of HPV infection was found in the inclusion bodies (5,13,14). The tumors regress spontaneously in chronological order with the increasing of fibrosis and decreasing in size and number of inclusion bodies (14). A link between the changes of inclusion and apoptosis may exist. Instead, no activated caspase 3, a key proteolytic enzyme in apoptosis, was observed from 11 immunohistochemical sections of IDF (15). On the basis of literature reviewing, two conclusions about the potential mechanisms of recurrence have been achieved (9,14). Firstly, residual tumor tissues may keep growing in this region due to incomplete excisions. Secondly, the exogenous trauma of surgery or biopsy may stimulate fibrosis proliferation to a certain extent. But in our case, the recurrence of IDF is based on complete surgical excision. The distinctive histopathologic phenomenon that koilocytes neighbor with eosinophilic inclusion bodies can not be explained by exogenous trauma. While HPV has been proved to be independent of the pathogenesis, the recurrence of IDF associated or concomitant with HPV infection has not been identified.
Low-risk HPVs (e.g., HPV-1, 4 and 6) are often related to some benign proliferative diseases, such as anogenital warts, verrucae vulgaris and verrucae plana. They are prevalent in 3.5% of adults and 33% of children (16). HPV-4 is the common cutaneous HPV types among children and adolescents, which belongs to γ papilloma virus types (17). In early regions of genome, HPV-4 E7 has the ability to degrade retinoblastoma and E8 can interact with calcium- and integrin-binding protein 1 (18). Recent studies have revealed that some of highly divergent γ types appear to be associated with squamous cell carcinoma (SCC) (19). Luisa et al. (20) analyzed the data from next generation sequencing and concluded that HPV-4 was abundant in actinic keratosis (AK), which is considered to be one of the precancerous states of SCC. In general, HPV-4 seems to participate in carcinogenesis to some extent. But it is unclear for their relationship between the potential carcinogenicity of HPV-4 and the recurrence of IDF.
The mechanism of recurrence is unknown. The recurrence of our case is associated or concomitant with HPV infection is still waiting for confirmation. But the unique case may provide a clue to the pathogenesis of the relationship between recurrent IDF and HPV infection. We believe that the report of IDF may enrich our understanding of the relationship between this rare disease and HPV.
Acknowledgments
Funding: We are thankful for the grants provided by the Maternal and Child Health Project of Jiangsu Province (F201717), the National Natural Science Foundation, China (No. 81573053), the Zhenjiang Social Project (No. SH2017005, No. SH2018032) and the Phylaxiology Project of Jiangsu Province (No. Y2018107).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-95
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-95). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from baby’s father.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agarwal H, Singh L, Sengar M. Infantile digital fibromatosis: Clues and pitfalls for cytological diagnosis. Cytopathology 2019;30:565-6. [Crossref] [PubMed]
- Gontijo JRV, dos Santos WF, Gontijo B, et al. Terminal osseous dysplasia presenting with intracytoplasmic inclusion bodies in digital fibromas. Pediatr Dermatol 2018;35:e353-6. [Crossref] [PubMed]
- Willemsen A, Bravo IG. Origin and evolution of papillomavirus (onco)genes and genomes. Philos Trans R Soc Lond B Biol Sci 2019;374:20180303 [Crossref] [PubMed]
- Reye RD. Recurring Digital Fibrous Tumors of Childhood. Arch Pathol 1965;80:228-31. [PubMed]
- Gaurkar SP, Nikam MP, Paithankar SS, et al. Infantile Digital Fibromatosis: A Rare Case Report. Indian J Dermatol 2016;61:222-4. [Crossref] [PubMed]
- Beckett JH, Jacobs AH. Recurring digital fibrous tumors of childhood: a review. Pediatrics 1977;59:401-6. [PubMed]
- Tomii K, Shimomura Y, Fujikawa H, et al. Case of infantile digital fibromatosis: Observation of its dermoscopic features. J Dermatol 2017;44:549-51. [Crossref] [PubMed]
- Agarwal H, Singh L, Gupta N, et al. Non-malignant fibroblastic/myofibroblastic tumors in pediatric age group: Clues and pitfalls to the cytological diagnosis. Cytopathology 2020;31:115-21. [Crossref] [PubMed]
- Liu B, Xu ZC, Bao PQ, et al. A case of infantile digital fibromatosis: differential diagnosis and treatment. Int J Dermatol 2014;53:e16-8. [Crossref] [PubMed]
- Spingardi O, Zoccolan A, Venturino E. Infantile digital fibromatosis: our experience and long-term results. Chir Main 2011;30:62-5. [Crossref] [PubMed]
- Marks E, Ewart M. Infantile Digital Fibroma: A Rare Fibromatosis. Arch Pathol Lab Med 2016;140:1153-6. [Crossref] [PubMed]
- Kramer A, Har-Shai Y, Metanes I, et al. The Use of Cryotherapy to Treat Infantile Digital Fibromatosis with a Functional Deficit: A Case Report. J Hand Surg Asian Pac Vol 2018;23:278-81. [Crossref] [PubMed]
- Zhu WY, Xia MY, Huang YF, et al. Infantile digital fibromatosis: ultrastructural human papillomavirus and herpes simplex virus DNA observation. Pediatr Dermatol 1991;8:137-9. [Crossref] [PubMed]
- Eypper EH, Lee JC, Tarasen AJ, et al. An Algorithmic Approach to the Management of Infantile Digital Fibromatosis: Review of Literature and a Case Report. Eplasty 2018;18:e19 [PubMed]
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma/fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol 2009;33:1-13. [Crossref] [PubMed]
- Al-Awadhi R, AlMutairi N, Chehadeh W. Prevalence of HPV genotypes in Adult Male Patients with Cutaneous Warts: a cross sectional study. Med Princ Pract 2020;29:458-64. [Crossref] [PubMed]
- Loenenbach AD, Poethko-Müller C, Pawlita M, et al. Mucosal and cutaneous Human Papillomavirus seroprevalence among adults in the prevaccine era in Germany - Results from a nationwide population-based survey. Int J Infect Dis 2019;83:3-11. [Crossref] [PubMed]
- de Jong SJ, Créquer A, Matos I, et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. J Exp Med 2018;215:2289-310. [Crossref] [PubMed]
- Pastrana DV, Peretti A, Welch NL, et al. Metagenomic Discovery of 83 New Human Papillomavirus Types in Patients with Immunodeficiency. mSphere 2018;3:e00645-18 [Crossref] [PubMed]
- Galati L, Brancaccio RN, Robitaille A, et al. Detection of human papillomaviruses in paired healthy skin and actinic keratosis by next generation sequencing. Papillomavirus Res 2020;9:100196 [Crossref] [PubMed]
Cite this article as: Hu HM, Long WG, Wang X, Li YM, Xu H. Recurrent infantile digital fibromatosis with HPV infection: a case report. AME Case Rep 2021;5:20.



