
Fiducial marker migration following computed tomography-guided placement in the liver: a case report
Introduction
Primary or oligometastatic liver tumors can be treated with radiation therapy with acceptable safety and a durable local control and survival benefit (1,2). However, diaphragmatic motion can create uncertainty in appropriately delineating the target. Such uncertainty may be mitigated by the insertion of a fiducial marker prior to radiotherapy in order to track respiratory motion and allow for more accurate dose delivery (3). Although fiducial markers improve the accuracy of liver-directed radiotherapy, placement of these markers may result in complications such as pain, post-procedural bleeding, pneumothorax, and less commonly migration (4). Fiducial marker migration is a relatively rare complication with scarce literature available regarding the location of such migrations and consequences. In prior series, reported rates of fiducial marker migration have ranged from 0.7% to 2.7% (5,6). At our institution, we placed fiducials in thirteen patients with hepatic neoplasms over the past year. We report the cases of two of these patients who experienced fiducial marker migration into the inferior vena cava (IVC) and right atrium after undergoing computed tomography (CT)-guided fiducial marker placement. We present the following cases in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-153).
Case presentations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients or their healthcare proxies.
Patient 1
A 79-year-old male with a 40-pack-year smoking history and asbestos exposure presented with right upper quadrant pain. He underwent a CT scan which demonstrated a large poorly defined hypervascular liver mass spanning the central aspect of the liver involving both the right and left lobes which measured 14 cm × 10 cm in cross-section extending to the capsular surface anteriorly. It also showed a tubular low-density structure in the left lateral segment of the liver which was thought to be a thrombosed branch of the portal vein or an occluded duct. He underwent a biopsy of the lesion which confirmed hepatocellular carcinoma (HCC) as well as a magnetic resonance imaging (MRI) scan which revealed a centrally located liver lesion measuring 14.2 cm.
After extensive multidisciplinary discussion, the patient was not deemed to be eligible for surgery or transplant due to portal vein involvement. Though radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and stereotactic body radiation therapy (SBRT) are all potential options to achieve local control and to increase disease-free survival, these also were not deemed to be ideal options for the patient given the degree of portal vein involvement by tumor. The tumor board recommendation was external beam radiation therapy (EBRT) with protons to the lesion, either as monotherapy or after TACE. He was advised to discuss TACE and systemic chemotherapy options as part of the comprehensive medical plan. Proton beam therapy was advised for this patient given that it can deliver a high dose to the target while sparing surrounding liver parenchyma, which may be cirrhotic in the setting of HCC, and has been found to provide excellent local control and survival rates in patients with HCC (7). Additionally, while SBRT is a common indication for fiducial marker placement due to the ablative doses delivered, fiducial markers are often used in proton therapy since many proton centers are only equipped with orthogonal kV imaging on which fiducial markers are easily visible as a surrogate for the tumor and its motion (8).
Thus, the patient underwent CT-guided fiducial marker placement within the liver tumor in preparation for definitive proton therapy. Interventional radiology performed fiducial marker placement and the team had a range of 3–30 years of experience. The fiducial markers used were Ion Beam Applications S.A. (IBA) VISICOIL fiducial markers which were launched at the American Society for Radiation Oncology (ASTRO) 2012 Annual Meeting conference and have two markers separated by a bioresorbable spacer. Twin VISICOIL fiducial markers measuring 0.50 mm × 0.5 cm separated by a 15-mm long non-stranded spacer were loaded in a 20-gauge pre-waxed brachytherapy needle. A deployment stylette was in the needle approximating the proximal edge of the two coils. Deployment was accomplished by withdrawing the needle while holding the stylette in place, thereby unsheathing the coils in the liver parenchyma. Review of the CT scan obtained during the deployment indicated that while the location of the distal coil was appropriate, the proximal coil was in the location of an unopacified hepatic vein. The proximal coil ultimately migrated through the hepatic vein and lodged in the junction of the hepatic vein and the IVC as demonstrated in Figure 1. The patient did not experience any sequelae from this migration.
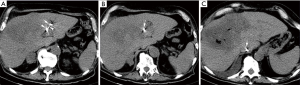
Patient 2
A 65-year-old female was initially diagnosed with right-sided colon cancer and underwent a hemicolectomy with final pathology revealing T3N2 disease. Prior to starting adjuvant chemotherapy, she underwent a positron emission tomography (PET) scan which revealed evidence of an isolated liver metastasis. After completing six cycles of FOLFOX, she was subsequently referred for resection of the liver lesion. However, during her operation, she was found to have a nodule along the peritoneum along the right paracolic region at the hepatic flexure which was found to be carcinoma on frozen section. Thus, the liver resection was aborted. She was subsequently re-started on additional chemotherapy from which she experienced symptoms of significant fatigue, memory changes, and intermittent abdominal pain.
She underwent a restaging CT scan which revealed a stable lesion in posterior inferior right hepatic lobe not significantly changed in size measuring 2.9 cm × 2.4 cm and stable size of omental nodules in the right upper quadrant with no other evidence of disease. She then underwent an omentectomy, partial hepatectomy of segment 6, cholecystectomy, resection of ileocolonic anastomosis, and biopsy of retroperitoneal and peritoneal nodules. Pathology revealed partly mucinous adenocarcinoma in the segment 6 liver lesion, consistent with metastasis from colonic primary with negative lymph nodes.
She continued on systemic therapy but was unable to tolerate further chemotherapy due to worsening memory. A subsequent PET-CT scan demonstrated increasing hypermetabolic uptake in the two known foci at the deep right posterior costophrenic angle and at the right lateral margin of the upper liver concerning for worsening disease as well as an additional focus of hypermetabolic uptake measuring 1.5 cm in maximum linear dimension with a standardized uptake value (SUV) of 5.0 and a questionable new lesion in the right hepatic lobe measuring 1.3 cm with an SUV of 5.4. A contrast-enhanced CT of the abdomen and pelvis demonstrated an irregular density in the right lobe of the liver (segment 7) corresponding to the area of abnormality on the PET-CT. Given that the patient had oligometastatic disease and could not tolerate further chemotherapy due to worsening memory, she was deemed to be an appropriate candidate for local treatment with SBRT.
In preparation for SBRT, twin fiducial markers were placed under CT-guidance into the tumors by our interventional radiologists with a range of 3–30 years of experience. Twin VISICOIL fiducial markers measuring 0.50 mm × 0.5 cm separated by a 15-mm long non-stranded spacer were loaded in a 21-gauge pre-waxed brachytherapy needle. A deployment stylette was in the needle approximating the proximal edge of the two coils. Deployment was accomplished by withdrawing the needle while holding the stylette in place, thereby unsheathing the coils in the liver parenchyma. Review of the CT scan obtained during the deployment indicated that while the location of the distal coil was appropriate, the proximal coil was in the location of an unopacified hepatic vein. The proximal coil ultimately migrated through the hepatic vein and into the right atrium as demonstrated in Figure 2. She did not experience any complications from the migration and two years have elapsed since she completed therapy.
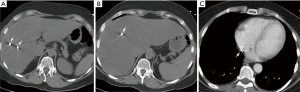
Discussion
Over the past 5 years, our institution has had a fiducial marker migration rate of five percent. We report the cases of unintentional migration of fiducial markers into the junction of the hepatic vein and IVC and into the right atrium which did not result in any toxicity to the patients in the aforementioned cases. While our study is limited by the fact that this is a single-institution report, its strength is that it reports the incidence of a rare complication of fiducial marker placement which has only scarcely been reported in the literature thus far. While fiducial marker migration in the prostate has been widely studied in the literature (9-11), the available data for the liver is more limited. With regard to fiducial marker migration from the liver, Shirato et al. reported one case of fiducial marker migration among 21 liver patients in which the marker migrated into the IVC and became trapped in a small vein at the hip without any adverse consequences (12). Moreover, Hennessey et al. reported a case of fiducial marker migration from the liver into the hepatic vein to the IVC which became lodged at the junction of the vena cava and the right atrium. He was referred to angiography to remove the coil and had no immediate or delayed complications (13). Finally, Kulkarni et al. reported one case of fiducial marker migration into the inferior interatrial septum without any adverse complications in his study of fiducial marker placement for liver lesions (6). Our case report is in accordance with these prior studies in that it demonstrates that there were no toxicities experienced as a result of fiducial marker migration from the liver. However, while the fiducial marker migrations did not result in toxicities in the aforementioned patients, it is important to recognize that such migrations can cause significant toxicity, especially in cases where individuals have a patent foramen ovale or pulmonary venous tributaries. For example, Farkas et al. reported a case of fiducial marker migration causing acute coronary syndrome after lodging into a posterior descending branch of the right coronary artery (14). Finally, fiducials may retract during deployment, leading to greater separation and deployment in a vascular structure. While the location of the distal marker may be in an optimal position during deployment, the proximal is often more difficult to visualize without CT. Thus, utilizing further imaging prior to deployment, such as contrast-enhanced CT or ultrasound-guidance via Doppler verification, may help to prevent or identify fiducial marker migration in a timely fashion. In particular, contrast-enhanced ultrasound allows for visualization of the liver microvasculature with exceptional sensitivity for small nodules and thin septations with the added advantages of providing dynamic real-time information and superior temporal resolution compared to CT (15). Moreover, a recent study has shown success with fiducial marker placement in the liver using volume navigation ultrasound techniques (16). The aforementioned advantages provided by contrast-enhanced and volume navigation ultrasound may help to prevent fiducial marker migration.
There have also been alternative strategies for motion management such as breath hold techniques and TACE lipiodol which have shown promise in liver radiotherapy. For example, a study by Mast et al. showed significant margin reduction with inspiration breath hold in liver SBRT without fiducial markers (17), while another study demonstrated the accuracy of expiration breath hold with image guidance in hypofractionated liver therapy (18). Finally, in patients who have undergone TACE lipiodol procedures, there is also evidence that residual lipiodol can be used as a surrogate marker in place of fiducials since it is easily visible on cone beam CT (19). The aforementioned techniques have demonstrated feasibility and efficacy and avoid the potential complications associated with fiducial marker placement. Nevertheless, fiducial marker placement is associated with improved local control in the setting of liver irradiation (20).
The current study augments prior literature by demonstrating that while fiducial marker migration is rare, it is a complication that merits further investigation to understand both its prevalence and potential consequences. Our case study highlights the complication of fiducial marker migration during CT-guided marker placement in patients with primary or oligometastatic liver tumors. Consideration of imaging prior to fiducial marker deployment may help to avoid unintentional migration of fiducials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-153
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-153). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients or their healthcare proxies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-91. [Crossref] [PubMed]
- Ohta K, Shimohira M, Murai T, et al. Percutaneous fiducial marker placement prior to stereotactic body radiotherapy for malignant liver tumors: an initial experience. J Radiat Res 2016;57:174-7. [Crossref] [PubMed]
- Roberge D, Cabrera T. Percutaneous Liver Fiducial Implants: Techniques, Materials and Complications. In: Mizuguchi Y. editor. Liver Biopsy in Modern Medicine. Croatia: InTech, 2011:107-16.
- Park SH, Won HJ, Kim SY, et al. Efficacy and safety of ultrasound-guided implantation of fiducial markers in the liver for stereotactic body radiation therapy. PLoS One 2017;12:e0179676 [Crossref] [PubMed]
- Kulkarni NM, Hong TS, Kambadakone A, et al. CT-guided implantation of intrahepatic fiducial markers for proton beam therapy of liver lesions: assessment of success rate and complications. AJR Am J Roentgenol 2015;204:W207-13 [Crossref] [PubMed]
- Mizumoto M, Okumura T, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys 2011;81:1039-45. [Crossref] [PubMed]
- Bertholet J, Knopf A, Eiben B, et al. Real-time intrafraction motion monitoring in external beam radiotherapy. Phys Med Biol 2019;64:15TR01 [Crossref] [PubMed]
- Poggi MM, Gant DA, Sewchand W, et al. Marker seed migration in prostate localization. Int J Radiat Oncol Biol Phys 2003;56:1248-51. [Crossref] [PubMed]
- Kupelian PA, Willoughby TR, Meeks SL, et al. Intraprostatic fiducials for localization of the prostate gland: monitoring intermarker distances during radiation therapy to test for marker stability. Int J Radiat Oncol Biol Phys 2005;62:1291-6. [Crossref] [PubMed]
- Delouya G, Carrier JF, Béliveau-Nadeau D, et al. Migration of intraprostatic fiducial markers and its influence on the matching quality in external beam radiation therapy for prostate cancer. Radiother Oncol 2010;96:43-7. [Crossref] [PubMed]
- Shirato H, Harada T, Harabayashi T, et al. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:240-7. [Crossref] [PubMed]
- Hennessey H, Valenti D, Cabrera T, et al. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep 2009;3:140. [Crossref] [PubMed]
- Farkas EA, Stoeckel DA, Nassif AS, et al. Intracoronary fiducial embolization after percutaneous placement for stereotactic radiosurgery. Ann Thorac Surg 2012;93:1715-7. [Crossref] [PubMed]
- Wilson SR, Burns PN, Kono Y. Contrast-Enhanced Ultrasound of Focal Liver Masses: A Success Story. Ultrasound Med Biol 2020;46:1059-70. [Crossref] [PubMed]
- Tokunaga K, Furuta A, Iizuka Y, et al. Utility of real-time image fusion technology in ultrasonography-guided fiducial marker implantation for stereotactic body radiation therapy for liver tumors. Acta Radiol 2020;284185120934479 [PubMed]
- Mast M, Kouwenhoven E, Roos J, et al. Two years' experience with inspiration breath-hold in liver SBRT. Tech Innov Patient Support Radiat Oncol 2018;7:1-5. [Crossref] [PubMed]
- Dawson LA, Eccles C, Bissonnette JP, et al. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys 2005;62:1247-52. [Crossref] [PubMed]
- Sioshansi S, Ding L, Fitzgerald TJ. SBRT Using Residual Lipiodol as Surrogate Fiducial for Image Guidance in the Treatment of Recurrent or Residual Hepatocellular Carcinoma. Pract Radiat Oncol 2013;3:S17. [Crossref] [PubMed]
- Feng M, Suresh K, Schipper MJ, et al. Individualized Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:40-7. [Crossref] [PubMed]
Cite this article as: Khullar K, Dhawan ST, Nosher J, Jabbour SK. Fiducial marker migration following computed tomography-guided placement in the liver: a case report. AME Case Rep 2021;5:15.


