Challenges in cannulation of left ventricular apex for temporary circulatory support: a case report
Introduction
Temporary circulatory ventricular assist device (VAD) support represents a significant improvement in the field of advanced heart failure. It has become a crucial therapeutic tool in the management of cardiogenic shock as a bridge to recovery, heart transplantation or a long-term implantable device (1). There is a growing experience with extracorporeal continuous-flow VADs such as the CentriMag System (Abbott, Intl.) as a short-term support. However, left ventricular drainage cannula insertion and secure techniques are still questionable in terms of safety for prolonged support duration. We describe a new simple technique that allows a safe placement of the inflow apical cannula of a temporary left ventricular assist device (LVAD).
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-19-191).
Case presentation
A 59-year-old patient was transferred to our center on circulatory support with peripheral veno-arterial extracorporeal membrane oxygenation (vaECMO) due to acute of myocardial infarction and acute biventricular heart failure. The patient underwent in the referral hospital percutaneous revascularization with a drug eluting stent in the left main coronary artery and the proximal left anterior descending artery but his clinical evolution was poor. When he arrived at our institution, signs of multiorgan dysfunction were documented. The echocardiogram showed severe left ventricular distention and failure. Right ventricular function was preserved. After an improvement of the clinical status, we decide to implant temporary LVAD CentriMag System as a bridge to decision.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Technique
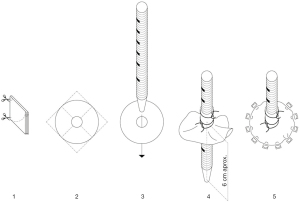
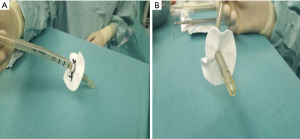
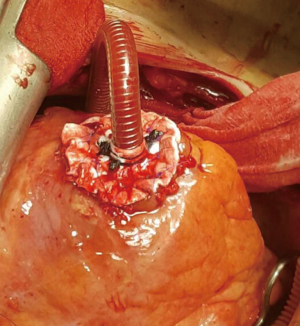
On the support of vaECMO median sternotomy was performed and the heart exposed. The tunneling of two cannulas was performed through a long subcutaneous tract to the skin through separate stab wounds at the left upper abdomen. A patch of soft Teflon felt was prepared with a central hole. Left ventricular cannula is then passed through the hole. The tip of the cannula is positioned approximately 4–6 cm to the felt square. The cannula is secured with three heavy silk ties placed in the proximal and central part of the Teflon. See Figure 1 (steps 1–5) and Figure 2. A cruciate ventriculotomy is performed and the inflow cannula introduced. Then, the Teflon square is sewn to the left ventricular apex using 12 isolated 4/0 prolene sutures supported with pledgets (Figure 3). This gives better fixation to apical myocardium (especially in case of fragile tissue after acute myocardial infarction) and secure intracavitary length of inflow cannula in a controlled mode, thus better than purse-string sutures only.
Outflow cannula is placed into aorta ascendens and secured using 2 double purse-string sutures with 4-0 polypropylene buttressed with pledgets passed through tourniquets. These tourniquets are folded and tied with another “0” silk tie on to the cannula at their entry point to aorta. Each cannula is sutured to the skin at three to four points. After de-airing and connection to the circuit LVAD is started with low speed gradually increasing support to a targeted flow >2.2 L/min/m2. A transesophageal echocardiographic examination showed inflow cannula with correct position and excellent drainage. At the end of surgery vaECMO was explanted after the confirmation of a stable right ventricular function and respiratory status. There were no technical problems, nor local hemorrhagic complications.
Follow-up
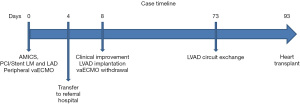
After the recovery of his multiorgan dysfunction and evaluation, the patient was included in the waiting list for heart transplantation. He remained stable on LVAD support and began to perform rehabilitation and ambulation in the ward. At day 65 it was necessary to change the external circuit due to the presence of fibrin deposition detected visually. He was finally transplanted after 85 days of LVAD support (Figure 4). Unfortunately, in the later postoperative period after transplantation, the patient presented an extensive cerebral infarct and died.
Discussion
Compared with the left atrial cannulation, apical cannulation of left ventricle provides better decompression with a better chance for partial of complete myocardial recovery. Left ventricular mechanical support results in deep volume unloading in the left ventricle with consequent reduction in ventricular size and shape leading to a phenomenon called reverses remodeling. In the published studies for CentriMag System double purse-string sutures with 2-0 or 3-0 polypropylene buttressed with pledgets passed through tourniquets were used to secure the apical drainage cannula of a temporary LVAD (2). However, after weeks of support this fixation technique may carry a significant risk of cannula dislodgment once patient became active during rehabilitation. Hence, others described the technique for left ventricular cannulation which requires construction of two grafts that were secured to the apex. Before the placement of the cannula into the left ventricular chamber it is mandatory to create an anastomosis between the graft and a square of soft Teflon or another type of patch (3,4). Here, suture lines of the fixation construction may play negative role and bring increased bleeding risk, especially in case of labile anticoagulation postoperatively. Manufacturer introduced in 2016 apical sewing ring and an “apical support cuff” to provide safe ventricular cannulation (5). Unfortunately, Abbott Company in Europe informed recently about withdrawal of this useful product from clinical use.
In our case we presented way of use of a patch of soft Teflon felt as analog of “apical support cuff”. It may help surgeons to fix apical inflow cannula appropriately, diminish risk for local surgical bleeding or cannula dislodgment and allow safe patient ambulation in case of prolonged temporary support. Disadvantage of Teflon use at left ventricular apex and induced material-related severe adhesion after month of support may be easily overcome by using any anti-adhesive membrane, such we did in our case. Further, in case of myocardial recovery, the neck of the patch may allow an easy direct closure of soft Teflon membrane after the withdrawal of the cannula without additional aggressive manipulation on apical myocardium.
Conclusions
Direct cannulation of the left ventricle apex using handmade soft Teflon “apical support cuff” improve surgical hemostasis and provide secure inflow length fixation during insertion of temporary LVAD. This simple, but effective, technique significantly diminishing risk of local bleeding complication, cannula displacement and facilitate transplant procedure or explantation.
Acknowledgments
We deeply thank Fernando Zaparain for his drawings.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-19-191
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-19-191). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- John R, Long JW, Massey HT, et al. Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. J Thorac Cardiovasc Surg 2011;141:932-9. [Crossref] [PubMed]
- Takayama H, Chen JM, Jorde UP, et al. Implantation technique of the CentriMag biventricular assist device allowing ambulatory rehabilitation. Interact Cardiovasc Thorac Surg 2011;12:110-1. [Crossref] [PubMed]
- Akhter SA, Raman J, Jeevanandam V. Technique for left ventricular apical cannulation for short-term mechanical circulatory support. Ann Thorac Surg 2010;89:994-5. [Crossref] [PubMed]
- Shen TC, Tsai KT, Hu CY, et al. Skirted Cannula Technique for Apical Cannulation in Implantation of Centrimag Left Ventricular Assist Device. Ann Thorac Surg 2016;101:2404-6. [Crossref] [PubMed]
- CentriMag® 34Fr Drainage (Venous)Cannula KitInstructions For Use (IFU). ©2016 Thoratec Switzerland GmbH-Document No. PL-0042, Rev. 09 (August 2016). Available online: https://manuals.sjm.com/~/media/manuals/product-manual-pdfs/4/a/4aaf0a40-abab-43a0-87f4-0deb41c30c5e.pdf
Cite this article as: Di Stefano S, Sarralde JA, San Román JA, Stepanenko A. Challenges in cannulation of left ventricular apex for temporary circulatory support: a case report. AME Case Rep 2021;5:32.