An adolescent female with a testosterone-secreting ovarian teratoma: a case report
Introduction
Ovarian teratomas, are the most common ovarian tumors seen in pediatric population with increased incidence with the onset of puberty, approximately 25 cases per 1,000,000 population per year between ages 15 and 19 years (1). The majority of teratomase are benign and typically asymptomatic but can also present with paraneoplastic syndromes which are caused by ectopic production of enzymes and hormones by the tumor cells leading to autoimmune conditions and endocrine phenomena. We present a case of a 16-year-old female who was diagnosed with a hormonally-active teratoma associated with virilization and menstrual irregularities
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-168).
Case presentation
A 16-year-old female with past medical history of asthma was referred to the pediatric gastroenterology clinic by her primary care physician for evaluation of chronic, intermittent abdominal pain. The pain was located in the periumbilical area and characterized as non-radiating and ‘burning’. The pain began several years prior and recurred several times a month without any identifiable exacerbating or alleviating factors. The patient denied any fever, nausea, vomiting, diarrhea, weight loss, or appetite changes. She reported that for the past 2 months, she noticed worsening bloating, which prompted her to seek medical attention. Her family history was remarkable for non-Hodgkin’s lymphoma in the maternal grandmother. Further history revealed that the patient had 3–4 menstrual spottings since she had her menarche at 13 years. Additionally, the patient noted deepening of her voice for the past year. She was also seen in pediatric endocrinology clinic. On physical exam, vital signs were stable. She had recessed temporal hairline. There was pain reproducible upon palpation of the periumbilical area. No rebound tenderness was noted. She had Tanner Stage V pubic hair development but Tanner Stage III breast development. Her external genitalia exam revealed clitoromegaly.
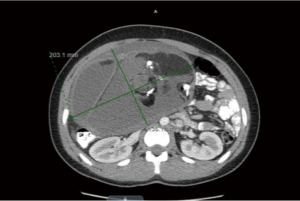
Due to voluntary guarding, an abdominal mass could not be appreciated. It was then decided to proceed with an abdominal ultrasound which revealed a 30×12×22 cm complex cystic mass of undetermined origin. The patient was referred to pediatric surgery for further evaluation. A CT of the thorax, abdomen and pelvis with contrast revealed a 13.6×20.3×31.1 cm mass rising from the left ovary of mixed attenuation containing areas of fat, calcifications and fluid which was concerning for teratoma or dermoid tumor (Figure 1). No lymphadenopathy or metastatic lesions were noted on the scan. Tumor markers associated with ovarian masses were obtained and revealed elevated CA-125 at 119.1 mcg/dL (<35). She had normal Beta-chorionic gonadotropin (β-hCG) <0.5 mIU/mL and Alpha fetoprotein (AFP) at 1.6 ng/mL (0.5–9). Lactic acid dehydrogenase and uric acid were also normal.
She had elevated serum total testosterone 313 ng/dL (9-58), and free testosterone 68.5 pg/mL (1.2–9.9), with normal sex hormone binding globulin (SHBG) 22 nmol/L (19–145). The rest of her androgens including dehydroepiandrosterone sulfate, 17-hydroxypregnenolone, and 17-hydroxyprogesterone were normal. Elevated testosterone level was most likely ovarian, origin. Thyroid studies including thyroid-stimulating hormone (TSH) and free thyroxine (FT4) were unremarkable and her karyotype was 46, XX.
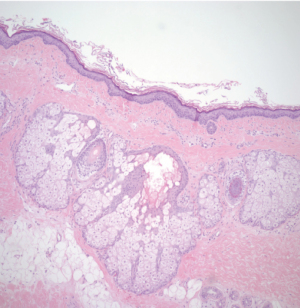
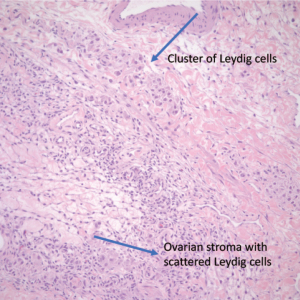
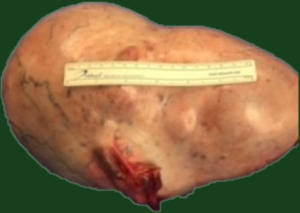
The patient underwent exploratory laparotomy and was found to have a 10 lb (4,545.45 g) cystic mass (Figure 2) originating from the left ovary which was excised with left salpingo-oophorectomy. Peritoneal fluid was also collected during the procedure and was negative for malignant cells. Pathology of the tumor revealed multiple cystic and solid areas with caseous material and hair, consistent with mature cystic teratoma. The histologic examination showed the presence of skin, respiratory epithelium, cartilage, and CNS tissue (Figures 3,4). In addition, well-differentiated Leydig cells with uniform polygonal cells, round nuclei, central nucleoli, abundant granular cytoplasm were identified (Figures 5,6) in ovarian stroma. There were no Sertoli cells identified on the specimen. Her two-week post operative serum total and free testosterone levels were found to be at normal female levels, suggesting that the presence of Leydig cells in her mature cystic teratoma was the source of testosterone. The patient started having regular menstrual cycles lasting for 5 days and associated with normal flow. Her post operative pelvic ultrasound was unremarkable.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013).
We were not able to obtain a written informed consent from the patient. The patient did not keep her follow up appointment. Multiple attempts made to obtain the consent via phone and mail. We were not able to reach family and the address on the file was undeliverable. There are no patient identifiable remarks in the report.
Follow-up and outcome
Five months after the surgery, she presented to the pediatric endocrinology clinic for follow-up. The patient was doing well, continued to have regular menstrual cycles, and had no signs of hirsutism or further virilization. A heterogeneous but predominantly hypoechoic lesion of the right ovary measuring 3.1×2.6×4.0 cm was found on repeat pelvic ultrasounds. Laboratory work-up revealed normal FSH, LH, estradiol, SHBG, serum total (39 ng/dL) and free (9.1 pg/mL) testosterone. Due to persistence of the mass on repeat pelvic ultrasounds, MRI of the pelvis with and without contrast was performed 6 weeks later and revealed resolution of the right ovarian mass which at that point was thought to represent a regressed complex ovarian cyst. However, the patient has been lost to follow up after her last imaging study.
Discussion
Ovarian neoplasms constitute 1% of childhood tumors and 10–20% are malignant (2-4). In contrast to the adult population, where the epithelial tumors are the most common type, germ cell tumors represent the majority of ovarian neoplasms (50–80%) in children and adolescents (5,6). The most common ovarian germ cell tumors are teratomas and consist of cells from the 3 germ cell layers (ectoderm, mesoderm and endoderm). They are classified as mature, and immature based on their histological characteristics. Mature ovarian teratomas contain only well-differentiated tissues, are benign, and represent up to 95% of the primary ovarian germ cell tumors (7). In most cases mature ovarian teratomas are typically asymptomatic and are diagnosed incidentally with imaging studies (usually pelvic ultrasound) that are performed based on other indications. Clinical manifestations that usually prompt medical attention include chronic nonspecific abdominal pain, constipation and/or palpable mass, which are usually caused by tumor expansion and compression of nearby structures. They may cause adnexal torsion, rupture, malignant transformation or infection(7). Androgen-secreting mature ovarian teratomas are rare, and the majority having been identified in postmenopausal women presenting with hirsutism, male pattern baldness, and virilization (8-11). Therefore, an androgen-secreting mature ovarian teratoma is extremely rare in adolescent years. To the best of our knowledge, our case is the fourth reported case on androgen secreting mature cystic teratoma in an adolescent. Our patient presented with similar symptoms, including hirsutism; virilization; and abdominal pain, that were seen in the previously reported cases (12-14). In all cases, including our patient, the presence of Leydig cells was accounted for the elevated testosterone levels.
Furthermore, in addition to Leydig cells, Sertoli-Leydig cell tumor, hilus-cell hyperplasia, and Brenner tumors were identified in ovarian teratomas as a source of androgen production (15-17). In our case, pathologic examination of the tumor did not reveal any Sertoli cells.
Different imaging studies including US, CT, and/or MRI can identify ovarian teratomas with their characteristic appearance (the presence of fat and calcified foci with a variable degree of a cystic component) (18). Differentiating mature and immature teratomas is challenging but is important especially in the pediatric population because it helps to determine treatment option and prognosis. Alotaibi and Navarro reported the predominance of a cystic component and a pure solid component in ovarian teratoma were significant differentiating factors between the mature and the immature teratoma (19). Additionally, the ovarian tumor markers CA-125, Alpha feta protein, and β-hCG can provide valuable preoperative information (20-22).
Until recently, the treatment of choice for ovarian teratomas in children and adolescents was open or laparoscopic oophorectomy (23). Today, given the very low risk of recurrence and malignancy, ovary-sparing surgery is a common approach in cases of localized mature teratoma with a clear border between the tumor and normal ovarian tissue and negative tumor markers (2,3,6). This surgical approach may have similar recurrence rates and it can theoretically increase fertility rates especially in patients with bilateral synchronous or metachronous neoplasms (7,24). However, laparotomy remains the treatment of choice in large masses, if malignancy is suspected and surgical staging is needed (7). Although it is usually done as an elective procedure, emergent intervention is warranted in cases of tumor-induced ovarian torsion (25). Definitive diagnosis of teratomas whether mature or immature is made based on the histologic characteristics of the tissue which will also determine its malignant potential, risk of recurrence, and will dictate further management and prognosis (23). Further studies are needed to define the best treatment approach in this patient population. In our case, we decided to proceed with oophorectomy because of large size of teratoma, elevated CA-125, and no definitive border between the tumor and the healthy ovarian tissue.
Most of the ovarian teratomas are benign and are associated with excellent prognosis (4). There is little evidence regarding follow-up care. Tumor markers can be used for monitoring of early recurrence especially if they were elevated pre-operatively. Frequent imaging with pelvic ultrasound is also recommended because recurrences may occur without elevation of tumor markers. In case of malignant tumors MRI and/or CT is usually performed after completion of the adjuvant chemotherapy and/or radiation therapy to evaluate for residual disease.
Conclusions
Androgen secreting ovarian teratomas should be included in the differential diagnosis when a female adolescent presents with abdominal pain, menstrual abnormalities and virilization. High index of suspicion is required for early diagnosis and management.
Learning points
- Ovarian teratomas have wide variety of clinical presentations.
- A triad of menstrual irregularities, virilization and abdominal pain should alert clinicians for androgen secreting ovarian teratomas.
- Ovary-sparing surgery should be considered if tumor markers are negative and there is a clear border between the tumor and healthy tissue in order to preserve future fertility in adolescents.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-168
Peer Review File: Available at http://dx.doi.org/10.21037/acr-20-168
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-168). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). We were not able to obrain a written informed consent from the patient. The patient did not keep her follow up appointment. Multiple attempts made to obtain the consent via phone and mail. We were not able to reach family and the address on the file was undeliverable. There are no patient identifiable remarks in the report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pediatric Treatment Editorial Board. Childhood Extracranial Germ Cell Tumors Treatment (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda MD2002. PDQ.
- Özcan R, Kuruoğlu S, Dervişoğlu S, et al. Ovary-sparing surgery for teratomas in children. Pediatr Surg Int 2013;29:233-7. [Crossref] [PubMed]
- Oltmann SC, Garcia N, Barber R, et al. Can we preoperatively risk stratify ovarian masses for malignancy? J Pediatr Surg 2010;45:130-4. [Crossref] [PubMed]
- Billmire D, Vinocur C, Rescorla F, et al. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. J Pediatr Surg 2004;39:424-9; discussion 424-9. [Crossref] [PubMed]
- Vaysse C, Delsol M, Carfagna L, et al. Ovarian germ cell tumors in children. Management, survival and ovarian prognosis. A report of 75 cases. J Pediatr Surg 2010;45:1484-90. [Crossref] [PubMed]
- Chabaud-Williamson M, Netchine I, Fasola S, et al. Ovarian-sparing surgery for ovarian teratoma in children. Pediatr Blood Cancer 2011;57:429-34. [Crossref] [PubMed]
- Łuczak J, Bagłaj M. Ovarian teratoma in children: a plea for collaborative clinical study. J Ovarian Res 2018;11:75. [Crossref] [PubMed]
- López-Beltrán A, Calanas AS, Jimena P, et al. Virilizing mature ovarian cystic teratomas. Virchows Arch 1997;431:149-51. [Crossref] [PubMed]
- Wu DH, McMurtrie DG, Hirsch SD, et al. Postmenopausal hyperandrogenism caused by a benign cystic teratoma: a case report. J Reprod Med 2008;53:141-4. [PubMed]
- Aiman J, Nalick RH, Jacobs A, et al. The origin of androgen and estrogen in a virilized postmenopausal woman with bilateral benign cystic teratomas. Obstet Gynecol 1977;49:695-704. [PubMed]
- Rutgers JL, Scully RE. Functioning ovarian tumors with peripheral steroid cell proliferation: a report of twenty-four cases. Int J Gynecol Pathol 1986;5:319-37. [Crossref] [PubMed]
- Rotenberg O, Shahabi S, Dar P. Testosterone-secreting mature ovarian teratoma causing severe virilization in an adolescent: sonographic and color Doppler characteristics. J Ultrasound Med 2009;28:85-8. [Crossref] [PubMed]
- Imperato-McGinley J, Peterson RE, Sturla E, et al. Primary amenorrhea associated with hirsutism, acanthosis nigricans, dermoid cysts of the ovaries and a new type of insulin resistance. Am J Med 1978;65:389-95. [Crossref] [PubMed]
- Hoffman JG, Strickland JL, Yin J. Virilizing ovarian dermoid cyst with leydig cells. J Pediatr Adolesc Gynecol 2009;22:e39-40. [Crossref] [PubMed]
- Subbaiah M, Dorairajan G, Gochhait D, et al. Virilization in a Postmenopausal Female Due to Androgen Secreting Ovarian Dermoid Cyst. J Midlife Health 2017;8:98-100. [Crossref] [PubMed]
- Seidman JD, Patterson JA, Bitterman P. Sertoli-Leydig cell tumor associated with a mature cystic teratoma in a single ovary. Mod Pathol 1989;2:687-92. [PubMed]
- Tsujimoto T, Takaichi M, Endo H, et al. A patient with diabetes and breast cancer in whom virilization was caused by a testosterone-producing mature cystic teratoma containing a Brenner tumor. Am J Med Sci 2011;341:74-7. [Crossref] [PubMed]
- Saba L, Guerriero S, Sulcis R, et al. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur J Radiol 2009;72:454-63. [Crossref] [PubMed]
- Alotaibi MO, Navarro OM. Imaging of ovarian teratomas in children: a 9-year review. Can Assoc Radiol J 2010;61:23-8. [Crossref] [PubMed]
- Göbel U, Calaminus G, Engert J, et al. Teratomas in infancy and childhood. Med Pediatr Oncol 1998;31:8-15. [Crossref] [PubMed]
- Marina NM, Cushing B, Giller R, et al. Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children's Cancer Group Intergroup Study. J Clin Oncol 1999;17:2137-43. [Crossref] [PubMed]
- Mann JR, Raafat F, Robinson K, et al. The United Kingdom Children's Cancer Study Group's second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 2000;18:3809-18. [Crossref] [PubMed]
- Templeman CL, Hertweck SP, Scheetz JP, et al. The management of mature cystic teratomas in children and adolescents: a retrospective analysis. Hum Reprod 2000;15:2669-72. [Crossref] [PubMed]
- Terenziani M, D'Angelo P, Inserra A, et al. Mature and immature teratoma: A report from the second Italian pediatric study. Pediatr Blood Cancer 2015;62:1202-8. [Crossref] [PubMed]
- Oltmann SC, Fischer A, Barber R, et al. Pediatric ovarian malignancy presenting as ovarian torsion: incidence and relevance. J Pediatr Surg 2010;45:135-9. [Crossref] [PubMed]
Cite this article as: Spyridakis E, Weidner B, Nguyen CK, Ergun-Longmire B. An adolescent female with a testosterone-secreting ovarian teratoma: a case report. AME Case Rep 2021;5:33.