Cardiac magnetic resonance in the diagnosis of the unusually detected acute myocarditis in the young people: a case report
Introduction
Myocarditis is a major cause of arrhythmic sudden cardiac death (SCD) in young athletes (1-3). Between 7% to 20% of SCD in young athletes is due to myocarditis. The proportion of SCD caused by myocarditis (SCD-myocarditis) is highly variable (between 1% and 14% among young people) due to the populations’ homogeneity and differences in the definition of SCD and post-mortem myocarditis. The etiology of myocarditis is heterogeneous, with viral infection being the most common cause worldwide, especially enterovirus, coxsackievirus B, parvovirus B-19, and human herpesvirus 6. Importantly, myocarditis recently has been reported as one of the cardiac complications of coronavirus disease 2019 (COVID-19) in athletes (4). This fact is particularly relevant since physical exercise during the acute phase of viral myocarditis may precipitate malignant ventricular arrhythmias. The diagnosis is based on a detailed medical history, physical examination, and complementary tests, such as appropriate blood work (e.g., troponin), 12-lead electrocardiogram (ECG), echocardiography, and eventually, cardiac magnetic resonance (CMR) (5).
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-24).
Case presentation
A 20-year-old male recreational soccer player was admitted to the cardiology unit after presenting with an episode of loss of consciousness in the context of respiratory infection without associated fever. The patient reports having woken up with symptoms of an upper respiratory tract infection, and after playing a soccer match, he developed dizziness and a headache. He then suffered vasovagal syncope without loss of sphincter control. No chest pain, nausea, vomiting, diarrhea, or other associated symptoms were reported.
The patient had no relevant family history of disease, with no cases of SCD. He had not previously reported palpitations, syncopal episodes, or dizziness associated with sports participation. No previous interventions were reported.
The physical examination on admission included a heart rate of 77 bpm and resting blood pressure of 134/76 mmHg;temperature: 36.7 °C; SpO2 98% on room air. Heart auscultation was normal. Symmetrical peripheral pulses were palpable. The patient had symmetrical carotid pulses, with no jugular engorgement at 45º, malleolar edema, or other heart failure signs. We also performed a blood test (Table 1). We also requested microbiological/serological tests.
Table 1
| Variables | Patient’s values | Normal values |
|---|---|---|
| Leukocytes/L | 24,200 | 4,500–11,000 |
| Neutrophils, % | 90.8 | 40–60 |
| Lymphocytes, % | 3.9 | 20–40 |
| Monocytes, % | 4.6 | 2–8 |
| Eosinophils, % | 0.4 | 1–4 |
| Basophils, % | 0.3 | 0.5–1 |
| Platelets, ×109/L | 229 | 150–450 |
| Hemoglobin, mg/dL | 14.8 | 13.5–17.5 |
| Hematocrit, % | 43.7 | 40–50 |
| Fibrinogen, mg/dL | 412 | 200–400 |
| Na, mEq/L | 135 | 135–145 |
| K, mEq/L | 3.4 | 3.5–5 |
| hs-cTnI, ng/mL | 0.11 | 0.060 |
| CRP, mg/dL | 1.9 | <0.3 |
hs-cTnI, high-sensitivity cardiac troponin I; CRP, C-reactive protein.
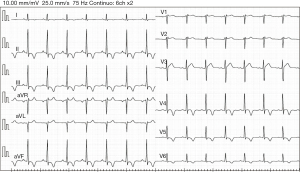
The 12-lead ECG showed sinus rhythm with a right bundle branch block (RBBB), with pronounced T-wave inversions (TWI) in inferolateral leads (Figure 1). The echocardiogram revealed non-dilated ventricular and atrial cavities, non-hypertrophic ventricular septum, and normal systolic and diastolic function. There was no pericardial effusion. Finally, a 24-hour Holter-ECG study was performed, which did not reveal any significant arrhythmia.
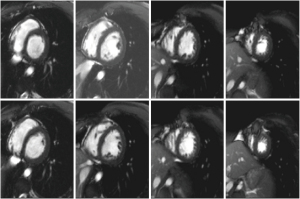
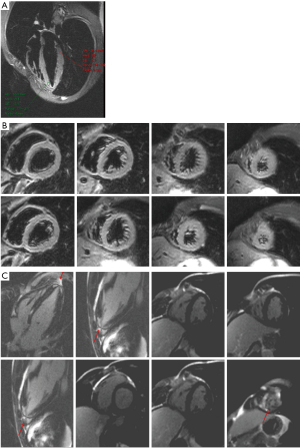
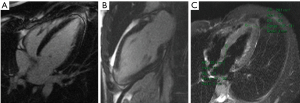
Viral serological test results were negative (i.e., adenovirus, enterovirus, toxoplasma Gondi, influenza virus A and B). A CMR was finally performed, revealing the absence of significant ventricular hypertrophy in a short-axis cine sequence of the left ventricle (LV) and right ventricle (RV) (Figure 2). However, an abnormal signal increase was observed at the apical level in the short-tau inversion-recovery (STIR) and 4-chamber sequences (Figure 3A,B). In addition, a pattern of apical fibrosis was observed in 4- and 2-chamber and short-axis late enhancement sequences for assessment of myocardial viability (Figure 3C), confirming the diagnosis of myocarditis.
The patient had a favorable evolution of symptoms, with abatement of the fever, an absence of heart failure signs or symptoms, arrhythmias or hemodynamic instability, and a good response to the symptomatic treatment administered (1 gram of acetaminophen every 8 hours). Given the good clinical evolution, the patient was discharged.
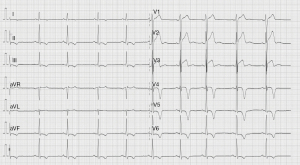
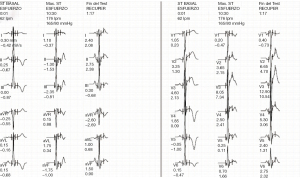
Sports practice was discouraged for six months, during which regular medical checkups were performed. In this regard, it is worth highlighting the last examination carried out two years after the initial presentation. The 12-lead ECG showed persistent T wave inversion in the inferolateral wall (Figure 4). The patient underwent an exercise stress test, showing anterolateral T-wave pseudonormalization with maximum effort (Figure 5), without induction of ventricular arrhythmias and negative for myocardial ischemia (ST elevation/depression). No arrhythmic episodes were found on a 24-hour Holter monitor. Finally, a control CMR showed apical fibrosis persistence in the 4- and 2-chamber late gadolinium enhancement (LGE) sequences without edema (Figure 6).The patient returned to exercise, playing soccer recreationally, and practicing light-to-moderate-intensity physical activity with high dynamic components.
All procedures performed in this case were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
A complete evaluation is recommended when myocarditis is suspected, including CMR (6). The case we have presented here is especially relevant in the COVID-19 era since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is among the potential causes provoking acute myocarditis in young athletes and other patients. In effect, SARS-CoV-2 infection has been associated with myocarditis. Current evidence indicates that approximately 20% of hospitalized patients suffered cardiac injury, with elevated levels of high-sensitivity troponins (hs-cTnI) (7). A meta-summary of cases reflected that CMR established the diagnosis of myocarditis in many patients, with the presence of edema and cardiac injury, highlighting the valuable role of CMR in these patients (8). In the COVID-19 era, CMR imaging will be a very useful and effective tool to assess cardiac involvement. LGE is the best predictor of mortality regardless of symptoms, functional class, ejection fraction, or LV end-diastolic volume (9). Ventricular scarring/fibrosis (as evidenced by LGE on the CMR), which may be missed by echocardiography, can cause SCD due to ventricular arrhythmias (10).
The finding of unexplained TWI on athletes’ 12-lead ECG should be thoroughly investigated by CMR imaging (11). In a study carried out by Schnell et al. (12), heart disease was established in 44.5% of 155 athletes with deep TWI (80% in lateral leads), with hypertrophic cardiomyopathy the most diagnosis (81%). Echocardiography was abnormal in 37 (53.6%) cases, while CMR revealed another 24 (16.6%) athletes with heart disease. Thus, CMR significantly improves the accuracy of diagnosing cardiomyopathy in athletes with marked repolarization alterations in the ECG and normal echocardiogram, as was also evidenced in the present clinical case.
CMR is currently the most widely used noninvasive imaging technique to confirm acute myocarditis, especially in hemodynamically stable patients during the first week of symptoms. CMR allows optimal myocardial tissue characterization, detecting inflammatory damage as necrosis and fibrosis in the form of LGE. Although the value of repeating the CMR at six months is unknown, as are its clinical and prognostic implications, it may be advisable to monitor the evolution of myocardial damage.
The optimal treatment of patients with acute myocarditis, and their prognosis, remain controversial. It is essential to highlight the need for prolonged monitoring with Holter-ECG or other devices regardless of ventricular function.
Due to the risk of exercise-induced arrhythmia, athletes with acute myocarditis should not return to competitive activity for 3–6 months after recovery from the acute process (13). The current sports cardiology guidelines of the European Cardiology Society (14) advise that athletes diagnosed with recent myocarditis should refrain from physical or sports activities while active inflammation persists, regardless of other factors (age, sex, LV systolic dysfunction). Likewise, it reinforces the previous recommendation of abstinence from moderate-to-high intensity exercise for a period of 3 to 6 months (5),highlighting the essential role of the CMR in the monitoring and evolution of this entity, together with a comprehensive evaluation after a complete recovery to assess the risk of exercise-related SCD.
Conclusions
CMR seems to be a useful diagnostic tool in athletes with suspected myocarditis, with excellent sensitivity for detecting inflammation, myocardial edema, and/or focal scarring. The distribution and extent of LGE with a non-ischemic pattern is an independent predictor of adverse cardiac events during follow-up.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-24
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-24). FSG serves as an unpaid editorial board member of AME Case Reports from Apr 2021 to Mar 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thiene G. Sudden cardiac death in the young: a genetic destiny? Clin Med (Lond) 2018;18:s17-23. [Crossref] [PubMed]
- Lynge TH, Nielsen TS, Gregers Winkel B, et al. Sudden cardiac death caused by myocarditis in persons aged 1-49 years: a nationwide study of 14 294 deaths in Denmark. Forensic Sci Res 2019;4:247-56. [Crossref] [PubMed]
- Bagnall RD, Weintraub RG, Ingles J, et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N Engl J Med 2016;374:2441-52. [Crossref] [PubMed]
- Phelan D, Kim JH, Elliott MD, et al. Screening of Potential Cardiac Involvement in Competitive Athletes Recovering From COVID-19: An Expert Consensus Statement. JACC Cardiovasc Imaging 2020;13:2635-52. [Crossref] [PubMed]
- Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2362-71. [Crossref] [PubMed]
- Sinagra G, Anzini M, Pereira NL, et al. Myocarditis in Clinical Practice. Mayo Clin Proc 2016;91:1256-66. [Crossref] [PubMed]
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-10. [Crossref] [PubMed]
- Ho JS, Sia CH, Chan MY, et al. Coronavirus-induced myocarditis: A meta-summary of cases. Heart Lung 2020;49:681-5. [Crossref] [PubMed]
- Gräni C, Eichhorn C, Bière L, et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol 2017;70:1964-76. [Crossref] [PubMed]
- Zorzi A, Perazzolo Marra M, Rigato I, et al. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ Arrhythm Electrophysiol 2016;9:e004229 [Crossref] [PubMed]
- Sharma S, Drezner JA, Baggish A, et al. International Recommendations for Electrocardiographic Interpretation in Athletes. J Am Coll Cardiol 2017;69:1057-75. [Crossref] [PubMed]
- Schnell F, Riding N, O'Hanlon R, et al. Recognition and significance of pathological T-wave inversions in athletes. Circulation 2015;131:165-73. [Crossref] [PubMed]
- Hurwitz B, Issa O. Management and Treatment of Myocarditis in Athletes. Curr Treat Options Cardiovasc Med 2020;22:65. [Crossref] [PubMed]
- Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17-96. Erratum in: Eur Heart J 2021;42:548-9. [Crossref] [PubMed]
Cite this article as: de la Guía-Galipienso F, García-González P, Fabregat-Andrés O, Quesada-Dorador A, Meyer-Josten C, Lavie CJ, Morin DP, Sanchis-Gomar F. Cardiac magnetic resonance in the diagnosis of the unusually detected acute myocarditis in the young people: a case report. AME Case Rep 2021;5:35.