
Colonic tuberculosis presenting as intestinal subocclusion in a patient with neuroendocrine tumor: a case report
Introduction
Tuberculosis is the third leading cause of mortality from infectious diseases worldwide. The most common presentation is pulmonary tuberculosis (PT), which usually corresponds to almost 85% of new cases in endemic regions. The incidence of extra-pulmonary tuberculosis (EPT) is variable and ranges from 7% in China to 53% in England and Wales. In Brazil, EPT rates are close to 14%, with intestinal presentation corresponding to only 2.2% of the EPT cases (available at https://www.saude.gov.br/images/pdf/2020/marco/24/Boletim-tuberculose-2020-marcas--1-pdf). EPT tends to affect a higher proportion of females, individuals with more than seven years of education, and patients with human immunodeficiency virus (HIV) infection or other comorbidities (1-3).
Intestinal tuberculosis (IT) is one of the clinical presentations of abdominal tuberculosis. The most common site of IT is ileocecal, followed by jejunum and colon. Isolated colonic involvement corresponds to 10.8% of the total IT cases. Multifocal disease in the colon may be present in 28% to 44% of the total cases of colonic tuberculosis (CT). The most common symptoms are abdominal pain and weight loss. Frequent abnormalities in colonoscopy are ulcerated lesions and stenosis, but polypoidal lesions have also been described (4-6).
The association of PT and oncological disease, particularly lung cancer, is well established (1,7). Meanwhile, the association of EPT and cancer is less clear. There are few reports on the association of CT and colorectal cancer. To the best of our knowledge, there are only two cases of neuroendocrine tumor (NET) and IT described so far. The first one published in 1988 (article in Japanese, only abstract available in English) described a NET of the ileum and healed tuberculosis of the ileum (8).The second case reported in 2016 described a NET of the appendix and tuberculosis of the cecum (9). Here we describe the first case on the coexistence of active CT and a NET of the small intestine. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-28).
Case presentation
A 61-year-old female was referred to our multidisciplinary NET outpatient clinic at the Clementino Fraga Filho University Hospital because of a 1-year history of abdominal pain, watery diarrhea and significant weight loss. There was no history of fever, night sweats, cough, vomiting, surgeries or comorbidities. Physical examination revealed a non-painful hepatomegaly. Her weight was 28 kg and her body mass index was 13.3 kg/m2. Other physical examination findings were unremarkable.
Investigation
The patient was referred to our hospital to perform a hepatic biopsy based on previous abdominal images that revealed multiple liver nodules and weight loss. The initial work-up for consumptive syndrome in order to search for chronic debilitating disorders revealed the following results: hematocrit [38.7% (reference range RR: 37–47%)], hemoglobin [12.6 g/dL (RR 12–16 g/dL)], white blood cells [8.200/mm3 (RR: 4.000–11.600/mm3)], glucose [66 mg/dL (RR: 74–99 mg/dL)], creatinine [0.7 mg/dL (RR: 0.5–1.0 mg/dL), albumin [2.2 g/dL (RR: 3.5–5.2 g/dL)],aspartate aminotransferase [20 u/L (RR: 10–35 u/L)], alanine aminotransferase [12 u/L (RR: 10–35 u/L)], and carcinoembryonic antigen [3.01 ng/mL (RR: <5 ng/mL)]. The HIV test was negative.
A colonoscopy was indicated and revealed a deep oval ulcer of 0.3 cm, with raised edges and regular contour in the ascending colon, a stenosis in the transverse colon, and an infiltrating tumor occupying most of the circumference of the descending colon, causing sub-total obstruction (Figure 1). Biopsy specimens were successfully performed in all lesions.
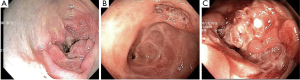
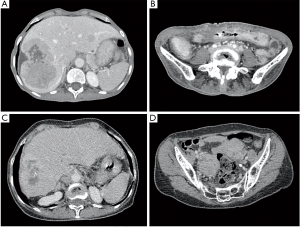
While the biopsy result was not available the patient’s symptoms progressed to bowel obstruction. An abdominal CT scan (ACTS) showed multiple large hepatic masses, spleen nodules, a pancreatic nodule, multiple enlarged intrabdominal lymph nodes in several sites, and a transverse colon stenosis (Figure 2). She underwent an urgent laparoscopy, with the supposed diagnosis of metastatic colon cancer. At surgery, extensive abdominal disease was reported, liver biopsy specimens were collected, and a transverse colostomy was performed.
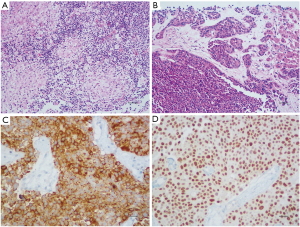
The histopathological examination of colonic lesions revealed the presence of granulomas with Langhans giant cells (Figure 3A). Mycobacterium tuberculosis (MT) culture was positive and confirmed the diagnosis of CT. The liver biopsy showed a grade 2 NET (Figure 3B), and the immunohistochemical study reactions were positive for chromogranin A (Figure 3C), synaptophysin, CD56, CD57, and showed an intense and diffuse homeobox protein CDX2 expression pattern (Figure 3D). The Ki-67 labelling index was 11.77%.
A chest computed tomography scan was performed for staging purpose. A bilateral apical pleural thickening was reported, but without scarring in the adjacent pulmonary parenchyma, which could suggest previous tuberculosis, if present. The patient denied a previous history of PT.
The patient was referred to our multidisciplinary NET outpatient clinic at the Clementino Fraga Filho University Hospital. Further investigation confirmed secondary bone involvement and an elevated urinary 5-hydroxyindoleacetic acid level (132 mg/24 h; RR: 2.0–9.0 mg/24 h). Although there was a pancreatic nodule, we conducted the case as an occult NET. The most probable primary site is the small bowel, because of the associated carcinoid syndrome and the CDX2 expression pattern. Thus, the pancreatic nodule was interpreted as a metastatic lesion. The patient also had spleen and myocardial metastasis (Stage IV cancer). Screening for multiple endocrine neoplasia type 1 was negative.
Treatment and follow up
She started anti-tuberculosis therapy with [rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E); RHZE], according to the manual of the Brazilian Ministry of Health, available at https://www.saude.gov.br/images/pdf/2020/marco/24/Boletim-tuberculose-2020-marcas--1-.pdf. Somatostatin analogue (SA) therapy was initiated one month later. The general condition of the patient improved, and her weight raised to 38kg, with no more flushing or diarrhea.
She underwent an elective laparotomy one year after the introduction of SA. The surgery goals were to reconstruct the intestinal transit, perform a cholecystectomy, and, if feasible, find and resect the primary lesion. There were multiple fibrosis and adherences that precludes an appropriate abdominal inventory. The surgeon performed the planned cholecystectomy and was able to remove the transverse ostomy. Functional imaging was not performed because it was unavailable at our hospital in that time.
The duration of the response to SA lasted 21 months, when the carcinoid syndrome symptoms returned and an ACTS confirmed disease progression. Thereafter, interferon therapy was added to SA with satisfactory control of the symptoms for another 9 months, while the patient waited for a planned 177Lu-DOTATOC therapy in another institution. The pre-treatment octreoscan showed myocardial metastasis in addition to the metastatic lesions mentioned above in bone, lymph nodes, liver, spleen, pancreas, and peritoneum. In fact, she received only two full doses with a four-week interval between them, but the treatment was interrupted due to a grade 4 thrombocytopenia.
The SA treatment was reintroduced, and resulted in controlled disease; however, a new disease progression was documented 18 months after the reintroduction of SA therapy. An ACTS showed an adequate control of hepatic, pancreatic, and splenic lesions, but an increase in the size of a mesenteric lymph node and enlarged ovaries [4.9 cm × 5.2 cm(right) and 4.5 cm × 3.9 cm (left)] (Figure 2C,D). Thus, treatment with interferon was started again in association with SA, with satisfactory control of the symptoms for another five months until her last visit. At this moment, we are planning a re-challenging with 177Lu-DOTATOC, with lower doses and a longer interval between each treatment cycle. Everolimus, another therapeutic option, is not available in the Brazilian public health system. On the last visit, the patient was oligosymptomatic, denied diarrhea or flushing, but complained of occasional abdominal pain.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
NET is considered a slow-growing cancer, usually with an indolent course of disease. We presented a case of a female patient, with multi-metastatic small bowel NET and carcinoid syndrome. The patient was cachectic and frail at diagnosis and had a severe coexistence of an opportunistic and symptomatic EPT. The cachexia could be a consequence of the untreated carcinoid syndrome and NET as well as of advanced tuberculosis.
Cancer cachexia is associated with several alterations in gut composition and function. These alterations include gut barrier dysfunction, altered ghrelin production and microbiota dysbiosis. The dysfunctional intestinal barrier may become more permeable due to the decreased expression of tight junction proteins and macrophage infiltration. The leaky barrier facilitates the entry of intact microorganisms or their fragmented components (10). This reinforces and justifies the association between CT and cancer cachexia in our patient. Another interesting aspect is that some of the main cytokines related to cancer cachexia have also been related to an increased risk of EPT, such as tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) (10,11).
A recent study explored the relationship between MT infection and lung cancer (7). First, the MT escapes the host’s immune defenses and establishes chronic and persistent inflammation, which is believed to create an appropriate microenvironment for the cancer development, the inflammation-related carcinogenesis. Additionally, experimental evidence in animals also revealed that chronic MT infection could lead to cell dysplasia in the lung (7). Therefore, we might suppose that synergistic mechanisms are involved in the association of both PT and EPT and cancer, and this may represent a public health concern in endemic areas of tuberculosis.
In fact, EPT incidence is increasing slightly, and is associated not only with HIV infection, but also with immunosuppressive drugs for rheumatic disease, solid organ and hematopoietic stem cell transplantation, chronic glucocorticoid therapy and end-stage renal failure and hemodialysis patients (1).
Abdominal tuberculosis may occur due to reactivation of a latent tuberculosis or by ingestion of MT via unpasteurized milk or undercooked meat. Approximately 20% of patients with abdominal tuberculosis also have PT. In our case, there were no alteration on chest computed tomography scan, which could be a source for the EPT reactivation. The most probable source of contamination in this case was the ingestion of contaminated food or milk (4). This is a scary situation considering that the patient lives in the second largest city of the country. The entire population is probably exposed to contaminated food or milk, but only susceptible patients develop the disease. In this scenario, we must reinforce food policies in the risky populations, listed above, to avoid these sources of contamination and prevent opportunistic IT.
The symptoms of CT are usually nonspecific, the most common are abdominal pain, weight loss and altered bowel habits. At colonoscopy, ulcerated lesions are the most frequently reported lesions. The most common radiological features are strictures, colitis and polypoidal lesions. Complications such as bowel perforation or fistula can be seen in up to 18% of the cases (4,5).
When considering all sites of IT, the most common complication is bowel obstruction. The obstruction may be caused by stenosis of the intestinal segment or by a mass effect, as occurred in our patient. In India, IT is the second most common cause of small bowel perforation (4,5).
In Brazil, IT corresponds to 2.2% of the EPT cases (2), while in the United States this percentage is four times higher, 9.4% (1). Therefore, in Brazil, this is an exceedingly rare situation, especially with bowel obstruction. Our case highlights this possible presentation in relation to the immunosuppressed population at risk and serves as a warning to physicians involved in the care of these patients.
We must also be attentive to the primary care of the patients with NET who usually have nonspecific symptoms and have a long delay before diagnosis. This delay allows progressive disease that may culminate in a presentation with a very advanced disease, as occurred in our patient.
As far as we know, this is the first reported case of the coexistence of CT and a small bowel NET with carcinoid syndrome. Although the patient was severely sick at the time of diagnosis, the evolution was favorable, and she is oligosymptomatic five years after diagnosis.
Conclusions
We described the first case of association between CT and small bowel NET along with carcinoid syndrome. The importance of this report is to raise the suspicion of EPT, in particular IT. Another point to be highlighted is the differential diagnosis of intestinal occlusion in cancer patients. In the presented case, the finding of IT causing the occlusion instead of a most probable cancer cause was unexpected and exceptional. The combination of contaminated food or milk along with gut alterations derived from cancer cachexia is a dangerous association for these patients with advanced disease. Further studies are necessary to improve the understanding of the physiopathology of the interaction among cancer, immunosuppression, and opportunistic infection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-28
Peer Review File: Available at https://dx.doi.org/10.21037/acr-21-28
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banta JE, Ani C, Bvute KM, et al. Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US 1998-2014. J Infect Public Health 2020;13:131-9. [Crossref] [PubMed]
- Ranzani OT, Rodrigues LC, Waldman EA, et al. Estimating the impact of tuberculosis anatomical classification on treatment outcomes: A patient and surveillance perspective analysis. PLoS One 2017;12:e0187585 [Crossref] [PubMed]
- Gomes T, Reis-Santos B, Bertolde A, et al. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect Dis 2014;14:9. [Crossref] [PubMed]
- Debi U, Ravisankar V, Prasad KK, et al. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol 2014;20:14831-40. [Crossref] [PubMed]
- Chakinala RC, Khatri AM. Gastrointestinal Tuberculosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2021.
- Chakinala RC, Farkas ZC, Barbash B, et al. Gastrointestinal Tuberculosis Presenting as Malnutrition and Distal Colonic Bowel Obstruction. Case Rep Gastrointest Med 2018;2018:2808565 [Crossref] [PubMed]
- Chai Q, Zhang Y, Liu CH. Mycobacterium tuberculosis: An Adaptable Pathogen Associated With Multiple Human Diseases. Front Cell Infect Microbiol 2018;8:158. [Crossref] [PubMed]
- Iso A, Ohtsuka H, Mizuma Y, et al. Carcinoid tumor of the ileum with intestinal tuberculosis--report of a case. Gan No Rinsho 1988;34:227-32. [PubMed]
- Ionescu L, Danila R, Vulpoi C, et al. Neuroendocrine tumor of the appendix and tuberculosis of the caecum in a patient with acute appendicitis. Acta Endocrinol (Buchar) 2016;12:368-9. [Crossref] [PubMed]
- Siddiqui JA, Pothuraju R, Jain M, et al. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim Biophys Acta Rev Cancer 2020;1873:188359 [Crossref] [PubMed]
- Webster AS, Shandera WX. The extrapulmonary dissemination of tuberculosis: A meta-analysis. Int J Mycobacteriol 2014;3:9-16. [Crossref] [PubMed]
Cite this article as: Brabo EP, Viana M, Caroli-Bottino A, Pannain VLN, Eiras A, Moraes AB, Vieira Neto L. Colonic tuberculosis presenting as intestinal subocclusion in a patient with neuroendocrine tumor: a case report. AME Case Rep 2021;5:36.


