Surgical and radiation treatment of a paravertebral malignant solitary fibrous tumor: a case report and literature review
Introduction
Solitary fibrous tumors (SFT) are rare neoplasms originating in the submesothelial mesenchymal cell layer (1,2), and comprise less than 2% of all soft tissue tumors (3). Historically identified as solitary lesions found only in the pleura, they are distinguished from the more common and diffusely-presenting malignant mesotheliomas, with just over 800 cases reported in the literature (2,4,5). Extrapleural disease is now seen most frequently; commonly reported sites include the peritoneum, retroperitoneum, viscera, pelvis, and head and neck (3,6). Within the pleura, 10–20% of SFTs are classified as malignant solitary fibrous tumors (MSFT), and have distinctive features including a more aggressive course characterized by frequent recurrences and metastases, larger size, symptomatic presentation, and increased FDG uptake compared to benign fibrous tumors. Diagnosis is classically made based on characteristic histologic findings of >4 mitoses/10 high-power fields, necrosis, atypia, and hypercellularity (1,2,6,7).
Complete surgical resection with at least 1–2 cm margins is the primary treatment modality for MSFT, though in contrast to benign SFT, local and distant failures are frequent and unpredictable (2,5,8,9). The role of adjuvant radiation is not well-established; its use is largely extrapolated from small reports of improved local control in the adjuvant or recurrent settings in both pleural and extrapleural MSFT—particularly in tumors greater than 5 cm in size or in those with positive margins (5,9-12). The paucity of data is clear however, with contradictory reports recommending against the use of adjuvant radiation as well, due to a lack of proven benefit (13). The existing literature focuses primarily on surgical management; adjuvant radiation—when offered—is described only briefly, and other than reporting dose ranges, robust data on radiation indications, techniques, and treatment volumes is notably absent (5,9-13). We present a case of a paravertebral MSFT, treated successfully with surgical resection followed by adjuvant radiation, and additionally provide the details of our radiation treatment plan and timeline for this rare malignancy.
We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-20-112).
Case presentation
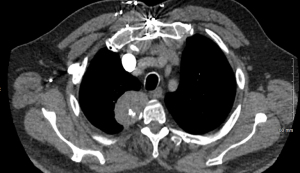
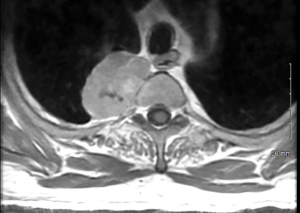
A 64-year-old man presented to the emergency department after developing a rash and right upper back and shoulder pain while recovering from a total hip arthroplasty. He had no history of trauma nor prior oncologic history. He had no other pertinent symptoms nor medical conditions and his physical exam was within normal limits. Chest X-ray revealed a 5 cm right pleural-based mass near the lung apex, and computed tomography (CT) pulmonary angiography further demonstrated a 5.2 cm ×4.0 cm ×4.0 cm right paraspinal mass with small areas of calcification (Figure 1). A thoracic spine magnetic resonance imaging (MRI) scan with gadolinium contrast medium showed a partially calcified, enhancing, right paravertebral mass measuring 5.4 cm at the level of T2–3, without spinal canal invasion (Figure 2).
Based on imaging findings, a benign neurofibroma was suspected, and he was taken for right video-assisted thoracoscopic surgical resection. Intraoperatively, a right paravertebral mass was identified and consistent with a typical neurofibroma. There was some minor adhesion of the lung to the mass which were easily dissected off with electrocautery. The mass was removed en bloc with the parietal pleura. The intercostal bundle at that level was clipped and ligated. There was no gross invasion of the ribs. The mass was then removed in its entirety, with successful reinflation of the lung and closure of all incision sites.
Grossly, pathology showed a well-circumscribed mass that appeared purple tan to brown, smooth and glistening on one surface, and red-brown and focally ragged on the opposing surface. The specimen weighed 53.6 grams, and measured 5.8 cm ×4.0 cm ×2.5 cm in size, and focally involved the inked cauterized edge of the largest fragment of tissue.
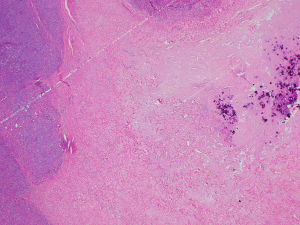
Microscopically, the tumor was composed of plump spindle cells with generally enlarged ovoid to elongated nuclei, nuclear hyperchromasia, and a small to moderate amount of amphophilic cytoplasm. The cells were arranged in vague fascicles with intermingled thick bundles of collagen. The tumor displayed high cellularity with scattered hypocellular fibrous zones, as well as areas of focal necrosis (less than 10% of mass), and dystrophic calcification (Figure 3). The tumor appeared generally well-demarcated with a surrounding fibrous capsule/pseudocapsule around the periphery, but focal infiltrative growth was noted. Mitotic figures numbered up to 14 in a count of 10 high-power fields; the Ki-67 labeling index for proliferation was variable, up to 10% in foci.
Immunohistochemically, the tumor was positive for CD34, Bcl-2, and STAT6, and negative for desmin, S-100, CAM5.2, EMA, and CD5. As there may be histologic overlap between MSFT and sarcomas, a fluorescence in situ hybridization analysis for SS18 (SYT) gene at the 18q11.2 locus, which is commonly associated with synovial sarcoma, was performed and was also negative. NAB2-STAT6 gene fusion was not tested. An additional outside slide review was performed as well, which agreed with the diagnosis of a malignant spindle cell neoplasm, compatible with MSFT, and confirmed to focally involve the inked margin.
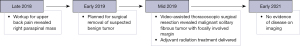
His post-operative course was uncomplicated. His case was subsequently discussed at our multidisciplinary thoracic tumor board in the presence of pathology, radiation oncology, and the operating surgeon. Based on the tumor board recommendations, he was referred for consultation with radiation oncology and thereafter, was recommended to undergo adjuvant radiation treatment to reduce the risk of local recurrence. He underwent a 4D CT simulation planning scan, and his treatment was planned by co-registering both his pre-operative imaging and a 6-week post-operative MRI of the thoracic spine with gadolinium, with the latter scan demonstrating a band of enhancing tissue along the posterior second intercostal space with decreased T2 signal. Upon radiology review, this enhancement could not be excluded as a potential focus of residual tumor. This area was then contoured as the gross tumor volume (GTV); a clinical target volume was added that encompassed the entire postoperative bed, including a 0.5 cm margin off of the GTV for subclinical disease spread, without expanding into the spinal cord. Lastly, a 0.5 cm planning target volume for uncertainties in treatment planning, delivery, or setup variation was added, to which a dose of 60 Gy in 30 fractions was delivered. He was treated using photon beam radiotherapy using two arcs delivered with a volumetric-modulated arc therapy. He tolerated treatment well with only mild grade 1 fatigue and dermatitis in the treated field during the final week of treatment (CTCAE v5.0). He experienced no acute pulmonary or gastrointestinal toxicity during treatment. At his last follow-up, there was no clinical or radiological evidence of recurrence at 21 months since surgery. A full timeline of his workup, treatment course, and post-treatment follow-up is depicted in Figure 4. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Although they may occur at any age, SFT of the pleura tend to appear in the sixth or seventh decades of life and distributed equally among men and women (5,8). Benign SFT are nearly uniformly cured with surgical resection alone (8). When rare recurrences do occur, they are typically re-resected successfully (14). Malignant varieties display a more unpredictable recurrence pattern and often portend a poorer prognosis (1,9). Most reports describe 5-year overall survival (OS) rates of 46–55% (1,3,15), with the exception of one report which that reported inexplicably higher 5- and 10-year OS rates each of 89% (16). However, from documented literature, the time to diagnosis, the inconsistency in extrapolation of the surgical technique and removal methods, the extent of resection as well as differences in histopathologic criteria for diagnosis of benign versus malignant SFT can all lead to this significant variability in reported outcomes (12,16).
Local recurrence rates range from 14−86% across multiple series, and while they most often occur within 24 months, late recurrences up to 17 years post-resection have been reported (5,10,12,15,16). Locoregional recurrences are most often managed with a repeat resection, however, disease recurrence remains the attributable cause of death in approximately 50% of such cases (5,16). Accordingly, the need for improvements in local control for MSFT is apparent. Drawing from the very limited available data, treatment doses of 50–65 Gy have historically been recommended for local control of what is typically a radiosensitive tumor, with doses at the higher end of the spectrum more commonly reserved for MSFT of the head and neck (9,11,12). Limited case reports support that pleural MSFT show a significant response to radiation treatment, though with the caveat that these were unresectable or recurrent tumors treated with radiation alone (17,18).
There are multiple challenges and barriers to conducting an overarching analysis of the role of radiation in the management of MSFT; specifically, the rarity of MSFT requires an exploration of more historical data with limited and/or antiquated radiation details (19), lack of consensus of indications for adjuvant radiation, inconsistencies in radiation delivery techniques as technology has evolved, varying doses based on intent and institutional preferences, and the widely heterogenous nature of reported cohorts. In a case report of a patient with a massive grossly resected MSFT, 55 Gy of adjuvant radiation was delivered due to tumor size, however critical details of margin status, radiation technique, fractionation, and treatment volumes were not included; notably, the patient was still disease-free for 26 months at the time of publication (9). In a larger review of 110 patients with pleural solitary fibrous tumors, only 15 were malignant, and four of these received adjuvant radiation with a reported survival range between 13 and 47 months (13). No additional information was provided regarding indications for radiation, treatment volumes, or doses; while the authors acknowledge that no conclusions could be drawn due to the limited sample size, they suggested that radiation may be indicated only for incompletely resected or inoperable patients (13). Overall, it is apparent that only a minority of patients with MSFT of the pleura receive adjuvant radiation, though with limited information on the determination of this treatment approach (13,16,20); it is less clear, however, whether this is due to an absence of data and uncertain indications for treatment, a lack of understanding of the potential role of radiation, or a combination of factors. In the present case, the decision to offer post-operative radiation to the patient was largely based on the patient’s focally positive margins; taking into account the additional consideration of the large tumor size, there was sufficient support for adjuvant radiation to a post-operative dose of 60 Gy in 30 fractions (9,11,16,21).
The diagnosis of MSFT is often not readily apparent based on imaging alone (8). In the paraspinal region, neurogenic tumors are often suspected initially as evidenced in our case with a preoperative presumptive diagnosis of a benign neurofibroma. CT imaging is classically the study of choice and often shows a well-demarcated, occasionally calcified, and usually heterogeneous mass (5,8). MRI may be subsequently acquired to further delineate the mass in relation to adjacent structures, and may also demonstrate increased signal intensity on T2-weighted images which can be indicative of the malignant fibrosis often present in MSFT (5).
All patients should undergo surgical resection as none of the imaging findings are ubiquitous or distinctive enough to differentiate a benign from a malignant tumor (9,16). Furthermore, drawing from population-based data, patients with MSFT of the thorax undergoing an oncologic resection had a greater than three- and five-fold reduction in deaths for both overall and cancer-specific survival, respectively (1). The ability to complete the surgical resection is paramount to reducing the risk of recurrence, which has even been reported to recur at thoracoscopic port sites due to contact metastases (10,16,22).
As with all case reports, this is limited in scope to an experience from a single patient, and therefore, it is difficult to draw any definitive conclusions, though a review of the available relevant literature was also conducted to support our treatment decisions. However, the unique circumstances of this case provide a strong platform for discussion and future management of other MSFT given the rarity of this disease and unclear treatment paradigms. Additionally, this report describes not only the surgical approach, but also a detailed radiation treatment perspective. This unique and underreported component of treatment provides additional support for consideration adjuvant radiation in this setting, which has otherwise been lacking in the published data for this tumor.
Conclusions
MSFT of the pleura are rare tumors that are usually difficult to diagnose and often have a locally aggressive clinical course. Surgical resection is the primary treatment modality, offering a significant survival benefit for completely resected tumors. Nevertheless, rates of local recurrence can be high and tend to be associated with certain high-risk features; however, the paradigm for adjuvant management of MSFT is not well established. Post-operative radiation treatment may improve rates of local control especially in the setting of concern for gross residual tumor or positive margins, subsequently preventing future morbidity and mortality.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-20-112
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-20-112). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Milano MT, Singh DP, Zhang H. Thoracic malignant solitary fibrous tumors: A population-based study of survival. J Thorac Dis 2011;3:99-104. [PubMed]
- England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640-58. [Crossref] [PubMed]
- Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002;94:1057-68. [Crossref] [PubMed]
- Sung SH, Chang JW, Kim J, et al. Solitary fibrous tumors of the pleura: surgical outcome and clinical course. Ann Thorac Surg 2005;79:303-7. [Crossref] [PubMed]
- de Perrot M, Fischer S, Bründler MA, et al. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285-93. [Crossref] [PubMed]
- Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology 2006;48:63-74. [Crossref] [PubMed]
- Kohler M, Clarenbach CF, Kestenholz P, et al. Diagnosis, treatment and long-term outcome of solitary fibrous tumours of the pleura. Eur J Cardiothorac Surg 2007;32:403-8. [Crossref] [PubMed]
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006;13:264-9. [Crossref] [PubMed]
- Filosso PL, Asioli S, Ruffini E, et al. Radical resection of a giant, invasive and symptomatic malignant Solitary Fibrous Tumour (SFT) of the pleura. Lung Cancer 2009;64:117-20. [Crossref] [PubMed]
- Cardillo G, Facciolo F, Cavazzana AO, et al. Localized (solitary) fibrous tumors of the pleura: an analysis of 55 patients. Ann Thorac Surg 2000;70:1808-12. [Crossref] [PubMed]
- Spitz FR, Bouvet M, Pisters PW, et al. Hemangiopericytoma: a 20-year single-institution experience. Ann Surg Oncol 1998;5:350-5. [Crossref] [PubMed]
- Yang XJ, Zheng JW, Ye WM, et al. Malignant solitary fibrous tumors of the head and neck: a clinicopathological study of nine consecutive patients. Oral Oncol 2009;45:678-82. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg 2009;88:1632-7. [Crossref] [PubMed]
- Okike N, Bernatz PE, Woolner LB. Localized mesothelioma of the pleura: benign and malignant variants. J Thorac Cardiovasc Surg 1978;75:363-72. [Crossref] [PubMed]
- Harrison-Phipps KM, Nichols FC, Schleck CD, et al. Solitary fibrous tumors of the pleura: results of surgical treatment and long-term prognosis. J Thorac Cardiovasc Surg 2009;138:19-25. [Crossref] [PubMed]
- Magdeleinat P, Alifano M, Petino A, et al. Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg 2002;21:1087-93. [Crossref] [PubMed]
- Liu M, Liu B, Dong L, et al. Recurrent intrathoracic solitary fibrous tumor: Remarkable response to radiotherapy. Ann Thorac Med 2014;9:245-7. [Crossref] [PubMed]
- Saynak M, Bayir-Angin G, Kocak Z, et al. Recurrent solitary fibrous tumor of the pleura: significant response to radiotherapy. Med Oncol 2010;27:45-8. [Crossref] [PubMed]
- Jha N, McNeese M, Barkley HT Jr, et al. Does radiotherapy have a role in hemangiopericytoma management? Report of 14 new cases and a review of the literature. Int J Radiat Oncol Biol Phys 1987;13:1399-402. [Crossref] [PubMed]
- Lahon B, Mercier O, Fadel E, et al. Solitary fibrous tumor of the pleura: outcomes of 157 complete resections in a single center. Ann Thorac Surg 2012;94:394-400. [Crossref] [PubMed]
- Bowe SN, Wakely PE Jr, Ozer E. Head and neck solitary fibrous tumors: diagnostic and therapeutic challenges. Laryngoscope 2012;122:1748-55. [Crossref] [PubMed]
- Nomori H, Horio H, Fuyuno G, et al. Contacting metastasis of a fibrous tumor of the pleura. Eur J Cardiothorac Surg 1997;12:928-30. [Crossref] [PubMed]
Cite this article as: Scher ED, Starnes S, Hagen MC, Daugherty EC. Surgical and radiation treatment of a paravertebral malignant solitary fibrous tumor: a case report and literature review. AME Case Rep 2021;5:38.