Multiple de novo gene variations in a progeroid phenotype case report: haploinsufficiency mechanisms
Introduction
Haploinsufficiency is a phenotype associated stage in where a single allele suffered an inactivation in a diploid organism (1). Beaudet and colleagues suggest in haploinsufficiency, a half-normal amount of gene product is insufficient to maintain a normal phenotype (2). The high representation of transcription factors and signaling molecules associated with haploinsufficiency syndromes suggests that signal transduction during embryonic development is particularly sensitive to gene copy number. Many reports have been found in medical literature in where the relationship between haploinsufficiency and genetic syndromes has been established. An example of these is the heterozygous inactivating mutations of the transcription factor PAX6 associated with an aniridia syndrome (3); mutations of GLI-3 are associated with Greig cephalopolysyndactyly syndrome (4) and mutations in ZNF-141 are associated with Wolf–Hirschhorn syndrome (5). On this case report, we present the case of 6-year-old patient with multiple de novo gene variations in a progeroid phenotype, associated with haploinsufficiency mechanisms. Previous cases have been reported regarding single-gene variations in the genes described on this case report, but no documentation exists regarding the combination of the multiple de novo gene variants reported here and its relationship with haploinsufficiency syndromes.
We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-25).
Case presentation
Case of a 6-year-old boy initially referred to genetic clinics due to clinical manifestations suggestive of a progeroid syndrome and developmental delay. The patient was initially evaluated on August 2014 at age 5 months and has been continuously evaluated at genetic clinic for phenotype characterization as well as to establish a genotype/phenotype correlation and the natural history of his anomalies. The patient was born to a 17-year-old female, and he was the product of an unremarkable pregnancy and delivery. Birth weight was 6 lbs. and 7 oz (22nd percentile) and his birth length were 21.5 inches (95th percentile). Presented respiratory complications at birth requiring indirect O2, elevated bilirubin, hypotonia and undescended testes at birth. Stay at NICU for three weeks due to poor sucking and respiratory difficulties. As he grew older severe developmental delay, hypotonia and failure to thrive were noted. Throughout the first three years of life seizures, hearing loss, and intellectual disabilities has been documented. The patient was initially consulted to University of California at Los Angeles genetic service via tele-medicine and the genetic team suggested progeria (Hutchinson-Gilford type) as a possible phenotype.
On April 2015, we requested a BRAF gene sequencing and del/dup studies that turned out negative. The child continued to present moderate to severe delay despite being receiving physical, occupational and speech therapies. His growth parameters normalized after the initial failure to thrive between 3 to 12 months of age. The patient presented abnormal EEG demonstrating abnormal background encephalic activity and evidence of epileptic discharges. Complete metabolic evaluation was done and was within normal values.
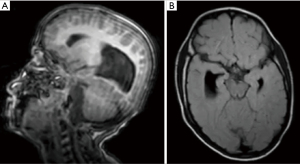
On March 2019, brain MRI showed callosal agenesis with bilateral colpocephaly, elongation and hypoplasia of the midbrain, parahippocampal atrophy with temporal horn dilatation, prominent Meckel’s caves bilaterally, widened bilateral internal auditory canals with Mondini malformation (incomplete partition anomaly with large vestibular aqueduct) and hypoplasia of the modiolus and possible vestibular aqueductal dilatation on the right, small cisterna magna pouch which may represent low-grade manifestation of Dandy-Walker continuum; and maxillary, ethmoid, and sphenoid sinus mucosal thickening and partial opacification (Figure 1). Head CT scan demonstrated plagiocephaly with flattening of the right occipital bone.
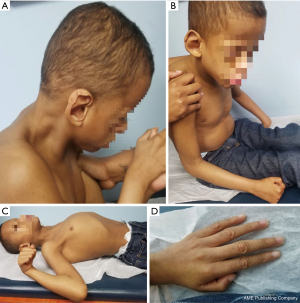
His examination on May 2020 was remarkable for dysmorphism (Figure 2) including plagiocephaly, low set and abnormally shaped ears, up slanted palpebral fissure, and hypoplastic alae nasi. His weight was 20 kg (25th percentile), height 117 cm (75th percentile), and a significantly low BMI of 14.6 kg/m2. His head circumference was 40.5 cm, which is two standard deviations below the 3rd percentile (microcephaly). His heart showed a regular rate and rhythm, his lungs were clear and showed no clinical evidence of visceromegaly. Neurologically, he presented decreased muscle tone and strength with gross motor delay and no speech. Additional dysmorphism (Figure 2) includes wrinkled skin, broad forehead with frontal bossing, sparse eyelashes in lower eyelid, short palpebral fissures of 1.8 cm (more than two standard deviations below the 5th percentile), upturned nares, thick lips, and right occipital plagiocephaly, overfolded helix and prominent anti-helix, protuberant chest, scaphoid abdomen, digitalized thumbs, and kyphosis due to low muscle tone. The outer canthal distance of 9 cm (97th percentile) and inner canthal distance of 3 cm (78th percentile); ear length of 6 cm (80th percentile), chest circumference of 65 cm (95th percentile), and wide space-apart inter-nipple distance of 17 cm (99th percentile). All other measurements were within the expected age range.
Metabolic evaluation including urine organic acids, plasma amino acids, ammonia, carnitine and acylcarnitine profiles were normal by May 2020. Blood WBC’s karyotype analysis and Chromosomal microarray studies were unremarkable showing a 47, XY and arr[hg19](1−22)×2, (XY)×1 results, respectively.
Echocardiogram was performed in May 2020 by 2-D, M-Mode, color flow and doppler examinations. There was a normal 4 chambers anatomy. Normal atrioventricular and ventriculo-arterial concordance. The left and right ventricular functions were normal. No evidence of atrial or ventricular septal defects was found. The 4 heart valves had normal anatomy and function with no stenosis or insufficiency. The aortic arch is left sided with normal branching. No aorto-pulmonary shunt was seen. In summary, a normal cardiac examination. Ophthalmology evaluation confirmed decreased vision with right eye +4 optic nerve pallor with deep cupping. Furthermore, spine X-ray is normal except for defect in the posterior elements of D11.
Whole exome sequencing analysis performed in August 2020 (Figure 3) demonstrated the following genetic changes with an autosomal dominant mode of inheritance. Heterozygous in the TTR gene with a sequence variant designated c.424G>A, which is predicted to result in the amino acid substitution p.Val142Ile. This variant, also referred to as p.Val122Ile and c.7356G>A using legacy nomenclature, has been reported to be causative for autosomal dominant hereditary amyloidosis (6-8). This variant is likely to be pathogenic, however we cannot exclude that this genetic mutation may be responsible for an abnormal protein folding resulting in a progeroid phenotype. TTR gene encodes for transthyretin, an evolutionarily conserved serum and cerebrospinal fluid protein. TTR protein aggregates in peripheral and autonomic nerves and heart, respectively; and senile systemic amyloidosis (SSA), a late-onset disorder in which wildtype protein deposits primarily in heart, but also in gut and carpal tunnel (9). For reasons that are unclear, the transthyretin protein abnormally begins to form protein deposits that may well be responsible for part if not all of the progeroid phenotype in this patient.

Heterozygous in RELN for a sequence variant designated c.4337A>G, which is predicted to result in the amino acid substitution p.Asn1446Ser. This variant is documented in 44 alleles of ~280,000 in the gnomAD general population database. Pathogenic variants in the RELN gene can cause autosomal dominant familial temporal lobe epilepsy. RELN encodes a largely secreted glycoprotein that is produced by specific cell types within the developing brain and activates a signaling pathway in postmitotic migrating neurons required for proper positioning of neurons within laminated nervous system parenchyma (10) and has been correlated with lissencephaly syndrome with cerebellar hypoplasia, seizures, midbrain hypoplasia, corpus callosum agenesis, language delay, autism spectrum disorder, and myoclonus dystonia. This patient presents with lissencephaly, cerebellar atrophy and severe developmental delay and learning disabilities, which are likely associated to the variation in this gene.
Heterozygous in MYH6 for a sequence variant designated c.679 G>A, which is predicted to result in the amino acid substitution p.Ala227Thr. This variant has not been reported in the literature and is reported in just 7 of ~251,000 alleles in the gnomAD general population database. MYH6 provides instructions for making a protein known as the cardiac alpha (α)-myosin heavy chain. Mutations in MYH6 have been reported and related with familial hypertrophic cardiomyopathy, dilated cardiomyopathy, atrial septal defect, and sick sinus syndrome. Our patient does not present any of these but is undergoing cardiology evaluation however we cannot exclude that he may develop later onset cardiomyopathy.
Heterozygous in PHIP for a sequence variant designated c.4849C>T, which is predicted to result in the amino acid substitution p.Leu1617Phe. It has been reported in 8 of ~280,000 alleles in the gnomAD general population. Pathogenic variants in PHIP can cause autosomal dominant developmental delay, intellectual disability, obesity, and dysmorphic features (OMIM #617991). Most documented variants have been de novo (11). PHIP acts as a substrate receptor in a ubiquitin ligase pathway and thus mediates substrate-specific proteolysis (12). Chung-Jansen Syndrome and histiocytosis-lymphadenopathy plus syndrome has been associated to mutations in this gene (11).
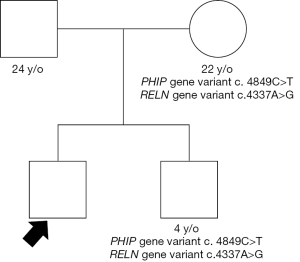
Heterozygous in the SYNE2 for a sequence variant designated c.13217C>T, which is predicted to result in amino acid substitution p.Ala4406Val. SYNE2 encodes nesprin-2, a member of the nuclear envelope spectrin-repeat family. Variants in these gene have been associated with Emery-Dreifuss Muscular Dystrophy 5 (OMIM #612999). This syndrome has some of the clinical manifestations that overlap with our patient’s phenotype. Patients’ mother and brother were analyzed for the genetic variants in MYH6, PHIP and RELN. Both had same variants on PHIP and RELN as our patient, with no apparent phenotypical consequences (Figure 4).
Some of the heterozygous recessive variations noted in this patient includes: KIAA0586 gene variant designated c.130 dup, which is predicted to result in a frameshift and premature protein termination. This variant has been reported to be causative for autosomal recessive Joubert syndrome (13). He is also heterozygous in the MESP2 gene for a variant designated c.258_261 del, which is predicted to result in frameshift and premature protein termination. Pathogenic variants in MESP2 are associated with autosomal recessive spondylothoracic (OMIM # 6086 dysostosis). MESP2 gene mutations have a higher prevalence in Puerto Rican population.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutions we are affiliated to and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We are presenting the case of a patient with multiple de novo autosomal dominant genetic variations. Patients’ father does not carry any of the genetic variations whereas some of the variants are present in mother and brother, who are asymptomatic, making them less likely to be of high impact on this phenotype. The complex phenotype presented in this patient is unlikely to be the result of a single gene variant, instead the result of multiple de novo dominant mutations, more specifically TTR, RELN and SYNE2 genes in conjunction with the loss of heterozygosity of at least the KIAA0586 and MESP2 genes.
Haploinsufficiency in genetics is usually applied as a model of dominant genetic phenotypes, in which a single copy of the standard (so-called wild-type) allele at a locus in heterozygous combination with a variant allele is insufficient back up for the necessary protein threshold to support the cell, tissue or organ mechanisms. Haploinsufficiency can occur through several ways including gene mutation and variations. Carriers of some recessive genes have been documented to present with phenotypical expressions.
The proteins products encoded by these genes are involved both in structural integrity, protein transport, and ciliogenesis processes. All these mechanisms are important for embryogenesis and cellular development and transduction. MYH6 provides instructions for making a protein known as the cardiac alpha (α)-myosin heavy chain. TTR gene encodes for transthyretin, an evolutionarily conserved serum and cerebrospinal fluid protein (6-9). RELN encodes a largely secreted glycoprotein that is produced by specific cell types within the developing brain and activates a signaling pathway in postmitotic migrating neurons required for proper positioning of neurons within laminated nervous system parenchyma and has been correlated with Lissencephaly Syndrome. This patient present with lissencephaly and severe developmental delay and learning disabilities which are likely associated to the variation in this gene. Mutations in MYH6 have been reported and related with Familial Hypertrophic Cardiomyopathy, Dilated Cardiomyopathy, and sick sinus syndrome (10). PHIP acts as a substrate receptor in a ubiquitin ligase pathway and thus mediates substrate-specific proteolysis. Variants in this gene are correlated with developmental delay, intellectual disability and dysmorphism (11,12). SYNE2 encodes nesprin-2, a member of the nuclear envelope spectrin-repeat family. Variants in this gene are related with autosomal dominant Emery-Dreifuss muscular dystrophy 5.
Given the complex phenotype and reserved prognosis on our patient, supportive management and palliative care is recommended. Levetiracetam is administered for seizure control. Physical, occupational and speech therapy is being received to prevent deterioration of his muscle mass or swallowing difficulties. The patient is also attending school as part of a special education class centered in children with moderate to severe developmental delays. Moreover, proteomic studies will help characterize the clinical manifestation of his phenotype and will provide better guidance to management strategies.
Conclusions
We propose haploinsufficiency and loss of heterozygosity mechanisms of gene expressions as part of the complex clinical presentation of this patient. Further proteomic studies may help to characterize the extent of each gene expression and the clinical manifestations in this progeroid phenotype as well as to determine if there are other genetic mechanisms involved. There may be other genetic mutations, not reported in the clinical Whole exome sequencing analysis, responsible for some of this patient phenotype.
Acknowledgments
We would like to acknowledge the usage of Genetic Diagnostic Group facilities and the San Jorge Children’s and Women’s Hospital Clinical Research Center.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-25
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-25). ASC reports payment or honoraria for Gaucher Disease and Hunter syndrome disease awareness lectures from Takeda pharmaceuticals, and he was on Advisory Board for Takeda Pharmaceuticals meeting (09/2020). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated, approached, and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutions we are affiliated to and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cook DL, Gerber AN, Tapscott SJ. Modeling stochastic gene expression: implications for haploinsufficiency. Proc Natl Acad Sci U S A 1998;95:15641-6. [Crossref] [PubMed]
- Beaudet AL. In: Harrison’s Principles of Internal Medicine. Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL, editors. New York: McGraw–Hill, 1998:365-95.
- Ton CC, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell 1991;67:1059-74. [Crossref] [PubMed]
- Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature 1991;352:539-40. [Crossref] [PubMed]
- Tommerup N, Aagaard L, Lund CL, et al. A zinc-finger gene ZNF141 mapping at 4p16.3/D4S90 is a candidate gene for the Wolf-Hirschhorn (4p-) syndrome. Hum Mol Genet 1993;2:1571-5. [Crossref] [PubMed]
- Penchala SC, Connelly S, Wang Y, et al. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci U S A 2013;110:9992-7. [Crossref] [PubMed]
- Jacobson DR, Gorevic PD, Buxbaum JN. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet 1990;47:127-36. [PubMed]
- Buxbaum J, Alexander A, Koziol J, et al. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010;159:864-70. [Crossref] [PubMed]
- Buxbaum JN, Reixach N. Transthyretin: the servant of many masters. Cell Mol Life Sci 2009;66:3095-101. [Crossref] [PubMed]
- Zaki M, Shehab M, El-Aleem AA, et al. Identification of a novel recessive RELN mutation using a homozygous balanced reciprocal translocation. Am J Med Genet A 2007;143A:939-44. [Crossref] [PubMed]
- Jansen S, Hoischen A, Coe BP, et al. A genotype-first approach identifies an intellectual disability-overweight syndrome caused by PHIP haploinsufficiency. Eur J Hum Genet 2018;26:54-63. [Crossref] [PubMed]
- Webster E, Cho MT, Alexander N, et al. De novo PHIP-predicted deleterious variants are associated with developmental delay, intellectual disability, obesity, and dysmorphic features. Cold Spring Harb Mol Case Stud 2016;2:a001172 [Crossref] [PubMed]
- Bachmann-Gagescu R, Phelps IG, Dempsey JC, et al. KIAA0586 is Mutated in Joubert Syndrome. Hum Mutat 2015;36:831-5. [Crossref] [PubMed]
Cite this article as: Hernandez-Hernandez C, Pascual J, Carlo S, Velez-Bartolomei F, Rodriguez E, Santiago Cornier A. Multiple de novo gene variations in a progeroid phenotype case report: haploinsufficiency mechanisms. AME Case Rep 2021;5:40.