
Rare co-inherited alpha-thalassemia minor and beta-thalassemia minor with heterozygous H63D mutation mistaken as iron deficiency anemia: a case report
Introduction
Anemia is extremely prevalent amongst the population, disproportionately affecting women more than men (1). Despite the various causes of anemia, iron deficiency anemia (IDA) remains the most common in women of reproductive age with reports of menorrhagia. Management of IDA is focused on replacing what is lost with iron supplementation. Even so, IDA is classified as a microcytic anemia alongside a few other diseases, one being thalassemia in which iron levels are normal or elevated and supplementation could lead to iron overload.
Thalassemia is a hemoglobinopathy predominantly found in Middle Eastern, South-East Asian, and Mediterranean populations that results from decreased or absent production of normal globin polypeptide chains, resulting in anemia. In adults, hemoglobin is composed of two alpha- and two beta-globin chains, known as hemoglobin A. Inherited in an autosomal recessive manner, thalassemia is due to a mutation in the alpha-globin (HBA1/HBA2) genes, or beta-globin (HBB) genes (2). Thalassemia’s are classified into alpha- or beta-thalassemia depending on the mutated globin chain inadequately produced and the severity of the disease is largely determined by the degree of chain imbalance (2). These alterations lead to impaired bone marrow erythropoiesis as well as destruction of red blood cells and their precursors resulting in anemia.
Clinical features of thalassemia vary depending on disease severity. Severe symptoms include failure to thrive, progressive jaundice, skeletal changes secondary to bone marrow expansion including frontal bossing, prominent malar eminence, maxillae hypertrophy, and long bone deformities. Milder forms of thalassemia are more subtle and present with fatigue and weakness. Patients suffering from mild to severe anemia often require regular blood transfusions which results in an increased risk of developing clinical manifestations of iron overload creating highly reactive oxygen species ensuing to organ damage. It is important to note that certain genetic diseases are associated with increased iron content, including hemochromatosis.
Current studies have only reported cases of a single thalassemia subtype, alpha- and beta-thalassemia, with a hemochromatosis H63D mutation. We present this rare case of co-inherited alpha-thalassemia minor and beta-thalassemia minor initially misdiagnosed as IDA in a reproductive aged female with a hemochromatosis H63D mutation. We will discuss the presentation and management of co-inherited thalassemia and the risk of iron overload, potential links to hemochromatosis and increased total body iron, in distinction to the importance of investigating all potential etiologies for anemia to prevent unwanted complications.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-40).
Case presentation
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 27-year-old, Asian female presented to the clinic with excessive lethargy and fatigue for the past 10 months, which she attested to studying rigorously for school exams. Patient acknowledged having a similar fatigue at her last wellness exam one year ago and was prescribed Ferrous Sulfate 325 mg daily supplementation due to a suspicion of IDA secondary to a history of heavy menstruations. Although her reports displayed an anemia with a low MCV, the patient declined therapy at that time due to abnormal labs, specifically regarding her urine and liver that subsequently lead to a hemochromatosis diagnosis after further investigation of elevated ferritin with genetic testing. She denied any changes in her appetite or weight, dyspnea on exertion, abdominal pain or growth, hematemesis, hematochezia, or melena. She also reported no history of easily bruising, skin discoloration, or periods of prolonged bleeding following an injury.
History
Patient has a heterozygous H63D gene mutation for hemochromatosis. She reports having a total of two phlebotomies prior to her visit today. She reports an ongoing history of asthma that is well controlled with an albuterol inhaler, environmental allergies currently being managed by immunotherapy, and a penicillin allergy. Patient denies alcohol, smoking or illicit drug use.
Family history
Family history is significant for type 2 diabetes mellitus (Mother and Father), Hypothyroidism (Mother), and Hypertension (Mother).
Physical exam
At presentation, patient’s vital signs were stable, with a blood pressure of 96/73 mmHg, a pulse of 94 beats/min, oxygen saturation of 99%, and a respiratory rate of 18 breaths/min. Upon physical exam, patient was noted to be alert, oriented, and well nourished. No abnormal physical characteristics noted. Cardiovascular examination findings were unremarkable. The lungs were clear to auscultation with no wheezing, rales, stridor, or signs of respiratory distress. Abdomen was soft, nontender, and without hepatosplenomegaly. The skin was cool and dry to touch with no noted rashes, erythema, urticaria, or bruising. Patient displayed no scleral icterus, jaundice, or pallor. The remainder of the examination findings were unremarkable.
Diagnostic tests
Laboratory tests disclosed the following results: red blood cells (RBCs), 4.71×106/μL (reference range, 3.58–5.19×106/μL); hemoglobin, 10.8 g/dL (reference range, 11.0–15.5 g/dL); hematocrit, 34.3% (reference range, 31.5–44.8%); MCV, 72.8 fL (reference range, 78.0–98.0 fL); mean corpuscular hemoglobin (MCH), 22.5 pg (reference range, 25.2–32.6 pg); mean corpuscular hemoglobin concentration (MCHC), 30.2 g/dL (reference range, 31.0–34.7 g/dL), red cell distribution width (RDW), 15.4% (reference range, 12.0–15.5%); reticulocyte count, <0.45% (reference range, 0.55–2.22%); white blood cells (WBCs), 7.32×103/μL (reference range 4.00–10.10 (×103/μL); platelet count, 227×103/μL (reference range, 140–425×103/μL); total bilirubin, 1.7 mg/dL (reference range, <1.2 mg/dL); aspartate aminotransferase (AST), 57 U/L [reference range <32 (U/L); and alanine aminotransferase (ALT), 77 U/L (reference range <33 (U/L)].
Iron studies disclosed the following results: serum ferritin, 28 ng/mL (reference range, 10–291 ng/mL); serum iron, 156 μg/dL (reference range, 37–145 μg/dL); iron saturation, 57% (reference range, 20–55%); and total iron binding capacity (TIBC), 275 μg/dL (reference range, 228–428 μg/dL).
Peripheral Smear disclosed the following results: microcytic and hypochromic red blood cells; normal leukocytes in number without overt cytologic abnormalities; differential: 52% neutrophils, 39% lymphocytes, 6% monocytes, 2% eosinophils, 1% basophils; and platelets are normal in both number and appearance.
Hemoglobin electrophoresis fractionations disclosed the following results: hemoglobin A2, 5% (reference range, 1.5–3.7%); hemoglobin A, 95% (reference range, 96–99%); hemoglobin F, 0% (reference range, 0%); hemoglobin S, 0% (reference range, 0%); and hemoglobin C, 0% (reference range, 0%).
Imaging
Ultrasound of the liver was unremarkable.
Genetic testing
Alpha-thalassemia minor with two of the four alpha-globin genes deleted, alpha3.7 and alpha4.2, consistent with alpha-thalassemia trait. The remaining alpha-globin genes are arranged in trans, with one copy located on each chromosome 16 homolog. Patient is negative for the Hemoglobin Constant Spring mutation.
Heterozygous, frameshift mutation of c.27dupG of the hemoglobin subunit beta (HBB) gene associated with beta-thalassemia minor.
Management
The patient’s anemia presents in the setting of normal ferritin, high iron saturation, and elevated A2 fraction is most likely in accordance to carrying the alpha-thalassemia minor, beta-thalassemia minor, and hemochromatosis H63D gene mutation. The initial diagnosis of IDA was inaccurate following iron studies displaying normal ferritin levels. The patient was started on folic acid 800 mg daily to enhance erythropoiesis. We will continue to monitor iron levels, iron saturation, ferritin, and hemoglobin along with the need for maintenance phlebotomies if serum ferritin levels are excessively elevated beyond normal values with adequate hemoglobin levels. Iron supplementation will be avoided unless ferritin is less than 20 ng/mL and the patient was advised to avoid iron-rich foods, such as red meat, dark green leafy vegetables, dried fruit, and iron-fortified products. Patient was strongly encouraged to undergo genetic counseling with her partner before childbearing in addition to seeking genetic testing in first- and second-degree relatives. Patient agreed to the plan and will follow-up in 6 weeks.
Discussion
Anemia is especially prevalent amongst women of reproductive age, affecting approximately 39% (3). Iron deficiency is the most common nutritional deficiency leading to anemia, contributing to more than 50% of all anemia cases and a quarter to a third of anemic cases in reproductive females (3). Identifying this nutritional deficiency is an important differential when investigating reasons for reduced hemoglobin levels in women of reproductive age, although additional etiologies must be examined as well due to disease progression and opposing managements, specifically those already at risk of iron overload.
In this case, we report a reproductive aged female positive for the hemochromatosis H63D mutation with a combination of alpha-thalassemia minor and beta-thalassemia minor that was initially mistaken for IDA. Co-inheritance of both thalassemia types is not uncommon, specifically alpha3.7 and alpha4.2 deletions with beta-thalassemia mutations (4) as presented in our patient. However, co-inheritance often poses an investigative challenge due to the clinical presentation (4,5). Coexisting thalassemias have the potential to blunt the severity and manifestations of the other mutations by causing a balance in globin chains. Additionally, both types of thalassemia will present with low mean corpuscular volume (MCV) and low mean corpuscular hemoglobin (MCH), while alpha-thalassemia will have a reduced or normal hemoglobin A2 as opposed to an elevation in beta-thalassemia (>3.5%) on hemoglobin electrophoresis (5). One type maybe masked by the other without specific genetic testing, as was identified in our case (Figure 1).
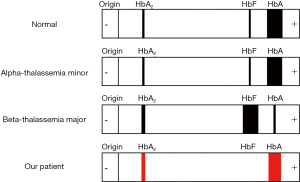
Management of thalassemia largely focuses on maintaining adequate hemoglobin levels with regular red blood cell transfusions. Although these measures will improve the thalassemia, each transfusion increases the risk of iron overload. One unit of packed red cells contains roughly 250 mg of iron which a patient will receive numerously throughout their lifetime (6). Although our patient does not require transfusions at this time, her risk of iron overload is still greater than the general population due to the ineffective erythropoiesis secondary to minor thalassemia mutations, which increases intestinal iron absorption (6) and a positive hemochromatosis H63D mutation with elevated liver enzymes and iron studies showing an increase in total iron and iron saturation.
Hereditary hemochromatosis is an autosomal recessive disorder resulting in excessive dietary iron absorption. This arises from alterations in genes regulating hepcidin synthesis, a liver-derived peptide that suppresses the release of iron from enterocytes, specifically HFE (7). Approximately 80% of hemochromatosis cases are associated with an HFE gene defect causing a tyrosine for cysteine substitution at amino acid position 282 (C282Y), most commonly seen in Caucasians (8). Another identified mutation includes H63D, of which H63D heterozygotes are not at risk of symptomatic iron overload progression as opposed to H63D homozygotes who can develop mild iron overload (8). H63D mutations are unlikely to result in iron overload unless seen as a compound heterozygote with C282Y (8). It is important to note that although H63D mutations are not at risk of iron overload, they can display minor elevations in serum iron measurements (8), as was seen in our patient’s iron studies. The standard treatment for hemochromatosis is opposite to thalassemia management. Phlebotomy is initiated to eliminate excess iron when serum ferritin levels far exceed normal range (7). Initial induction is more aggressive to quickly reduce serum ferritin levels to 20–50 ng/mL in order to restore safe iron levels in the blood (7), as seen in our patient. Subsequently, maintenance phlebotomies maintain a target serum ferritin level between 50–100 ng/mL to avoid an iron deficiency with lower serum ferritin levels paradoxically leading to further hepcidin depression and increased iron absorption (7).
Co-inheritance of a thalassemia subtype, either alpha or beta alone with a hemochromatosis H63D mutation have been identified in various studies. Multiple investigations into the connections between isolated H63D mutations and iron overload in beta-thalassemia have been conducted with conflicting conclusions; some studies support an effect while others deny any relation possibly due to sample size, different ethnicities, sex-ratio, and degree of thalassemia severity (9). Despite opposing beliefs, all can agree that ferritin levels are elevated in beta-thalassemia major patients with an H63D variant as opposed to thalassemia carriers without the variant (9). Even so, a minority of thalassemia carriers have the potential to develop iron overload, depending on the presence of other acquired or genetic factors and the use of long-term iron supplementation mistaken for IDA (10). Pokhrel et al. describes the case of a 73‐year‐old male with cirrhosis, secondary to hemochromatosis resulting from beta-thalassemia minor and a homozygous H63D mutation on long-term iron supplementation for IDA.
Withal, studies have only reported cases of a single thalassemia subtype with a hemochromatosis H63D mutation and our search of the literature did not find any previous reports of combined alpha- and beta-thalassemia with a heterozygous H63D mutation as seen in our patient. Although the clinical presentation of our patient and laboratory values are stable, the course of inheriting all three diseases together is unknown. Frequent monitorization of hemoglobin levels and iron studies will be conducted to follow this rare presentation and prevent life-threating iron overload.
Conclusions
Anemia is highly prevalent amongst the population and identifying the etiology is most important but may be challenging, especially in reproductive aged females. The coexistence of alpha- and beta-thalassemia is not uncommon and neither is a single thalassemia subtype with a hemochromatosis H63D mutation, but the inheritance of all three diseases has yet to be reported. Co-inherited thalassemia and hemochromatosis alone warrants a concern of developing into an iron overloaded state, but the effects of combined alpha- and beta-thalassemia with a heterozygous H63D mutation may inflate the risk beyond reported studies. In addition, patients taking supplemental iron must be monitored using routine serum iron studies to prevent unnecessary consequence leading excessive iron deposits, such as in misdiagnosis IDA.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-40
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol 2011;4:177-84. [Crossref] [PubMed]
- Angastiniotis M, Lobitz S. Thalassemias: An Overview. Int J Neonatal Screen 2019;5:16. [Crossref] [PubMed]
- Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci 2019;1450:15-31. [Crossref] [PubMed]
- Shahid S, Nadeem M, Zahid D, et al. Alpha thalassemia deletions found in suspected cases of beta thalassemia major in Pakistani population. Pak J Med Sci 2017;33:411-6. [Crossref] [PubMed]
- Li J, Xie XM, Liao C, et al. Co-inheritance of α-thalassaemia and β-thalassaemia in a prenatal screening population in mainland China. J Med Screen 2014;21:167-71. [Crossref] [PubMed]
- Marengo-Rowe AJ. The thalassemias and related disorders. Proc (Bayl Univ Med Cent) 2007;20:27-31. [Crossref] [PubMed]
- Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 2010;139:393-408, 408.e1-2.
- Bacon BR, Adams PC, Kowdley KV, et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:328-43. [Crossref] [PubMed]
- Yang J, Lun Y, Shuai X, et al. Late-onset Hemochromatosis: Co-inheritance of β-thalassemia and Hereditary Hemochromatosis in a Chinese Family: A Case Report and Epidemiological Analysis of Diverse Populations. Intern Med 2018;57:3433-8. [Crossref] [PubMed]
- Pokhrel NB, Khanal S, Chapagain P, et al. Hemochromatosis in a β-thalassemia minor patient with H63D homozygous mutation: A case report. Clin Case Rep 2020;8:2341-5. [Crossref] [PubMed]
Cite this article as: Chaudhry AF, Malik Z, Shegos CJ. Rare co-inherited alpha-thalassemia minor and beta-thalassemia minor with heterozygous H63D mutation mistaken as iron deficiency anemia: a case report. AME Case Rep 2022;6:4.


