
A multidisciplinary approach to prolonged extracorporeal membrane oxygenation for acute respiratory distress syndrome due to coronavirus 2019—case report
Introduction
The novel coronavirus 2019 (COVID-19) has become a threat to global health. Since initial detection of the virus, there have been more than 110 million confirmed cases of COVID-19 worldwide (1). In China as of March 1, 2020, the mortality rate was 3.6% and elsewhere was 1.5% (2). The observed case fatality rate in the United States is 1.8% as of February 2021 (3). COVID-19 can cause fatal comorbidities such as acute respiratory distress syndrome (ARDS) (4). The prevalence of ARDS caused by COVID-19 is approximately 8.2% (5). Extracorporeal membrane oxygenation (ECMO) can be used as salvage therapy in severe cases of ARDS and has been used in patients with ARDS due to COVID-19 (6). Although there is limited evidence on the outcomes of these patients supported with ECMO, the results of the studies published during the COVID-19 outbreak show that the mortality rate is approximately 82.3% (7). Additional studies have shown that the pooled odds of mortality for ECMO versus conventional therapy in patients with severe ARDS due to COVID-19 was not significantly different, with a mortality rate of 94.1% in the ECMO patients and 70.9% in conventional therapy patients (8). There are a limited number of studies outlining the effect of multidisciplinary care teams in patients with respiratory failure undergoing ECMO. One study explored the efficacy of a multidisciplinary team approach to ECMO in patients with COVID-19 with a support team comprised of physicians, nurses, perfusionists, and bioethicists and found that it contributed to the 80% survival rate of among five patients who underwent ECMO (9). Another retrospective study of patients with severe acute respiratory failure who underwent ECMO found that the mortality rates were significantly decreased in patients who had a multidisciplinary support team compared to those who did not (10). It also reported a reduced number of cannula problems and cardiovascular events as well as improved 1-year mortality. The following is a case of recovery from COVID-related ARDS with single organ failure on ECMO for 91 days and discharged from the hospital after 159 days. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/acr-21-51).
Case presentation (Figures 1-4)
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (IRB #11D.185) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
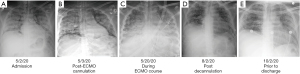
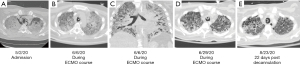
A 35-year-old male presented to a community hospital on 05/02/2020 complaining of three days of fever, dry cough, shortness of breath, and malaise. He had a past medical history of type 1 diabetes and was a never smoker. His height was 160 cm, weight 69 kg, body surface area 1.75 cm2 and body mass index 26.9. In the emergency department, the patient was found to be hypoxic and tachycardic on a nonrebreather mask, but with a stable blood pressure. COVID-19 testing by nasopharyngeal swab was positive for SARS-CoV-2 by polymerase chain reaction (PCR). His whole blood lactate was 13.7 mmol/L, venous blood gas was pH 7.06, white blood cell count 19.8 B/L with neutrophilic predominance, D-dimer 6,620 ng/mL, ferritin 1,165 ng/mL, and C-reactive protein 33 mg/dL. Chest X-ray (CXR) showed bilateral opacification (Figure 1A). An electrocardiogram showed sinus tachycardia and S1Q3T3 pattern indicative of right heart strain. A bedside point of care echocardiography showed flattening of the interventricular septum also consistent with right pressure overload, with preserved left ventricular function (ejection fraction 75%). Given the concern for pulmonary embolism (PE) and subsequent hemodynamic compromise, he was taken for a CT angiogram which showed bilateral consolidations and pneumomediastinum but was negative for PE (Figure 2A). The patient remained hypoxic and in respiratory distress, requiring intubation on the day of admission. He was transferred to our hospital a few hours later for higher level of care with inhaled epoprostenol and empiric antibiotics.
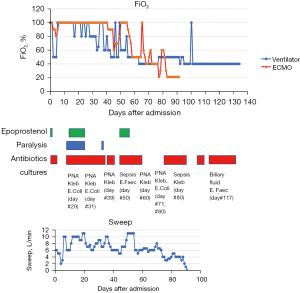
Upon arrival to the medical intensive care unit (MICU) he was noted to have rhonchi bilaterally with crepitus above the clavicle on exam. He was on ventilatory support of assist-control/volume control (AC/VC) at a rate of 30, tidal volume 420 cc, FiO2 100% with PEEP 12 mmH2O for an ideal body weight of 57 kg. His corresponding arterial blood gas was pH 7.35, PaCO2 65, and PaO2 75. Out of concern for ventilator associated barotrauma and severe ARDS, the patient was placed on veno-venous extracorporeal membrane oxygenation (VV ECMO) with an 18-French return cannula in the right internal jugular vein and a 22-French drain cannula in the right femoral vein on the day following MICU admission (05/03/2020). He was transferred to the cardiovascular intensive care unit (CVICU) primarily for ECMO management. He was started and maintained on high ECMO support with FiO2 100% and sweep 6 L/min, flow 4.3 L/min, and with ventilator pressure control settings of: inspiratory pressure 15 mmH2O, rate 15, PEEP 15 mmH2O, and FiO2 100% with returned tidal volume range of 19 to 479 mL (Figure 3). His CXR appeared unchanged from prior (Figure 1B). He completed five doses of hydrocortisone 50 mg intravenously (IV) on 05/04/2020 and one dose of tocilizumab on 05/05/2020 after consultation with Infectious Disease (ID) and Rheumatology.
The patient had increasing sedation and analgesia requirements which prompted consults to the Acute Pain Management Service (APMS), Psychiatry, and Palliative Care teams. Endocrinology was consulted as well in the setting of poorly controlled blood sugars and steroid administration.
Shortly after ECMO cannulation, he developed bleeding of the nasopharynx, urethra, and ECMO cannulation site associated with coagulopathy which was concerning for disseminated intravascular coagulopathy (DIC); thus, Hematology, Otolaryngology (ENT), Urology, and Acute Care Surgery (ACS) were consulted and he received multiple transfusions of packed red blood cells (PRBC), fresh frozen plasma (FFP), cryoprecipitate, and platelets, as well as desmopressin and vitamin K. In addition, he intermittently required norepinephrine for hypotension to maintain a mean arterial pressure greater than 60 mmHg. Nutrition was consulted for total parenteral nutrition support.
Multiple bedside bronchoscopies were performed in the setting of ARDS and concern for mucus plugging (Figure 1C). The bronchoscopies did not reveal clots or bleeding, but one bronchoalveolar lavage on 05/21/2020 was positive for Klebsiella pneumoniae. The patient later developed multiple episodes of bacteremia, specifically, with pan-sensitive Enterococcus faecalis and Klebsiella pneumoniae. His pneumonia and blood stream infection eventually cleared with antibiotic treatment guided by an ID consultation.
After a multidisciplinary discussion with CVICU, Pulmonary, and ID physicians, the patient was given another short trial of steroids to reduce inflammation of the lung noted on CT scan despite the Klebsiella pneumoniae and Escherichia coli in sputum (Figure 2B,2C). He received dexamethasone 20 mg IV daily from 06/09-06/17/2020 for an indication of ARDS. This was tapered to 10 mg and his course was completed seven days later (Figure 2D).
The ECMO oxygenator was exchanged on 06/25/2020 when the team noted oxygenator exhaustion with hypoxia, hypercapnia, and visible clots in the oxygenator. There was a discussion about potential exchange of VV ECMO circuit to single double lumen VV ECMO cannula (AvalonTM cannula) catheter, though this was deferred. His course was further complicated by a pneumothorax which required a right sided chest tube on 06/11/2020 and another on 08/10/2020 to the left chest by thoracic surgery. Despite the best efforts of the social work and case management teams, lung transplantation was declined because of socioeconomic reasons, namely an inability to apply for United States insurance. He underwent a tracheostomy placement on 07/06/2020 which led to improvement in his compliance on the ventilator. The patient was COVID negative per nasopharyngeal swab PCR as of 06/17/2020 with positive COVID antibodies on 06/19/2020 and 09/12/2020. His lung function slowly improved, and his ECMO sweep gas was discontinued 07/31/2020. He was decannulated on 08/01/2020 after 91 days on ECMO (Figure 1D) and then transferred to the MICU. While in the MICU, a plan was developed by Psychiatry, Palliative Care, Pharmacy, and Nursing to taper his analgesia and sedation to oral medications. Psychiatry also assessed him due to the primary team’s concern for delirium and noted he was having mild insomnia and delirium but was without signs of depression or post-traumatic stress disorder (PTSD).
He was downgraded from the MICU to the floors on 08/19/2020, day 107. There he was found to have acute cholecystitis and small bowel inflammation which was subsequently treated with antibiotics and a percutaneous cholecystostomy drain by Gastroenterology and ACS. He later developed an aspiration pneumonia also treated with a course of antibiotics (Figure 2E). He was weaned off the trach collar and his tracheostomy was decannulated on 9/28/2020, his percutaneous gastrostomy tube was removed on 10/03/2020, and his percutaneous biliary drain was removed on 10/09/2020.
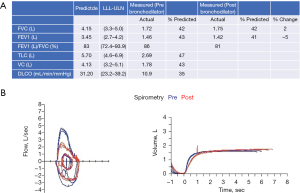
He was discharged to a rehabilitation center on continuous two liters nasal cannula oxygen with physical and occupational therapy (Figure 1E). He did not require any medications for his respiratory status. He had subsequent pulmonary function testing on 12/1/2020 which showed severe restrictive lung disease and severely reduced diffusion capacity (Figure 4A,4B). He was seen in the pulmonary office one time after he was discharged and then followed up in the Post-Intensive Care Syndrome (PICS) Clinic. There he was evaluated by the multidisciplinary team and deemed to have PTSD with a Trauma Screening Questionnaire score of 6 and depression based on a Beck’s Depression Inventory score of 14. He had no personal or family history of depression prior to discharge. He was referred to a local clinic that provided primary care and behavioral health in his native language. As of six months after discharge from the hospital, he has been at home with intermittent oxygen requirements and not yet back to full-time work.
Discussion
This is a case of COVID ARDS with isolated single-organ dysfunction and prolonged ECMO support requiring a multidisciplinary approach to care, ultimately resulting in clinical recovery.
One study of 213 hospitals worldwide showed that in patients with COVID-19 who received ECMO, both estimated mortality 90 days after ECMO and mortality in those with a final disposition of death at discharge were less than 40% (11). There are studies supporting the idea that while VV ECMO successfully manages patients with severe isolated lung injury, once patients develop acute kidney injury, they are likely to further develop multi-organ dysfunction. This includes hepatic and hematological complications and has been shown to lead to inferior survival (12). If this patient had experienced multi-organ failure, it is likely the team would have withdrawn care at an earlier date.
This patient did have bleeding which was concerning for hematologic dysfunction and DIC but it was mitigated by transfusions of FFP, PRBC, vitamin K, and cryoprecipitate. He also had two events of sepsis during ECMO, likely secondary to pneumonia, which was addressed with broad spectrum antibiotics (Figure 3). He was on vasopressors for hypotension, although this may have been due to a systemic inflammatory reaction due to ECMO and/or COVID infection as his subsequent echocardiograms showed preserved left ventricular function and only mild right ventricular dysfunction. Despite these complications, his renal, liver functions were maintained as was his mental status. There was serious consideration of lung transplantation, but this was deferred due to socioeconomic barriers.
It has been posited that VV ECMO should be reserved for patients in whom the potential benefits outweigh the risks and for whom a meaningful recovery from COVID-19 is a possibility (13). Single-organ failure may be a good measure of meaningful recovery in these patients. In those COVID ARDS patients with intact renal, metabolic, hematologic, and cardiovascular function, ECMO should be strongly considered.
Femoral-internal jugular VV ECMO is among the standard cannulation methods for a patient with COVID-19 (14). Compared to Avalon cannula placement which requires multiple healthcare providers, bedside fluoroscopy, and echocardiography for placement, femoral-internal jugular cannulation can be done with just a single surgeon and one assistant. Due to the high risk of the spread of COVID-19 to healthcare providers, and lack of vaccination at the time, Avalon cannula placement was deferred for this case.
This case highlights the many clinical and social complications medical teams encounter when treating COVID ARDS patients on ECMO and the inherent need for a multidisciplinary team. We took a concerted multidisciplinary approach to this patient, including physicians from Rheumatology, ID, GI, ACS, Palliative Care, Pulmonary and Critical Care, Rehabilitation Medicine, Endocrine, Psychiatry, Thoracic Surgery, APMS, ENT, and Hematology in his care plan. In addition, we utilized Pharmacy to titrate his analgesia and sedation to an oral regimen so he could transition to the general medical floors. Nursing titrated his many drips based on clinical status and signs of withdrawal. Case Management and Social Work tried to obtain eligibility for lung transplantation while on ECMO. This case clearly underscores the need for effective multidisciplinary collaboration to ensure patient survival and management of severe COVID ARDS particularly on ECMO. Notably, this patient had a prolonged post-ECMO course with cholecystitis, inflammatory bowel disease, and aspiration pneumonia, all of which were able to be managed on the general medical floors.
The patient has had a difficult recovery period, suffering from depression and PTSD which he did not have prior to his discharge from the hospital. It is possible that his prolonged ECMO course and subsequent delirium left him vulnerable to these sequelae. There are several small studies which assess the long-term neurocognitive and psychiatric outcomes of patients with severe ARDS who underwent ECMO. One prospective cohort study of 40 patients with severe ARDS compared those patients to those not treated with VV ECMO and found that VV ECMO treatment did not worsen long term cognitive and neuropsychological outcomes in severe ARDS survivors (15). In contrast, the PRESERVE trial, which was designed to identify factors associated with death post-ICU discharge in patients with ARDS who underwent VV ECMO, also assessed anxiety, depression, and PTSD frequencies; it found that a health-related quality of life (HR-QoL) evaluation in 80% of the 6-month survivors revealed persistent physical and emotional-related difficulties, with anxiety, depression or PTSD symptoms reported by 34%, 25% or 16%, respectively (16). A study subsequent to this also evaluated HR-QoL in a different cohort of these patients and their caregivers; it found a consistent risk of psychological morbidity (anxiety, depression, and PTSD at 42%, 42%, and 47%, respectively), worse than in the PRESERVE study (17). These studies support that patients with ARDS managed with VV ECMO may have good survival but often do suffer from long term psychological impairments, including PTSD. The PICS clinic should play a role in the care of these patients after discharge to identify those with such sequelae and to refer to mental health professionals.
Conclusions
This case is an example of a multidisciplinary team approach to ECMO leading to successful treatment of a patient with COVID-19 ARDS. Implementation of formal multidisciplinary teams for these patients on ECMO and comparison of mortality before and after may be warranted. Furthermore, single-organ failure may be an important predictor of meaningful recovery in patients on prolonged VV ECMO due to COVID-19 ARDS. In patients with COVID ARDS with intact renal, metabolic, hematologic, and cardiovascular function, ECMO should be strongly considered. Further retrospective studies of survival of patients with COVID-19 ARDS on ECMO are required.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/acr-21-51
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/acr-21-51). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (IRB #11D.185) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- COVID-19 Dashboard by the center for systems science and engineering at Johns Hopkins University. Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- Baud D, Qi X, Nielsen-Saines K, et al. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis 2020;20:773. [Crossref] [PubMed]
- “Mortality analyses: mortality in the most affected countries.” Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/data/mortality
- Li X, Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care 2020;24:198. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Li X, Guo Z, Li B, et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019 in Shanghai, China. ASAIO J 2020;66:475-81. [Crossref] [PubMed]
- Ñamendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung 2020;49:348-9. [Crossref] [PubMed]
- Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J Crit Care 2020;58:27-8. [Crossref] [PubMed]
- Nagaoka E, Arai H, Ugawa T, et al. Efficacy of multidisciplinary team approach with extracorporeal membrane oxygenation for COVID-19 in a low volume ECMO center. Artif Organs 2021;45:1061-7. [Crossref] [PubMed]
- Na SJ, Chung CR, Choi HJ, et al. The effect of multidisciplinary extracorporeal membrane oxygenation team on clinical outcomes in patients with severe acute respiratory failure. Ann Intensive Care 2018;8:31. [Crossref] [PubMed]
- Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020;396:1071-8. [Crossref] [PubMed]
- Devasagayaraj R, Cavarocchi NC, Hirose H. Does acute kidney injury affect survival in adults with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation? Perfusion 2018;33:375-82. [Crossref] [PubMed]
- Hoyler MM, Kumar S, Thalappillil R, et al. VV-ECMO usage in ARDS due to COVID-19: Clinical, practical and ethical considerations. J Clin Anesth 2020;65:109893. [Crossref] [PubMed]
- Dovidio J, Hirose H. Special consideration for ECMO cannulation and decannulation for COVID 19 patient. In: Firstenberg MS. editor. The History of extra-corporeal membrane oxygenation (ECMO): from start to COVID. Nova Science Publishers, 2021:131-50.
- Sylvestre A, Adda M, Maltese F, et al. Long-term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients. Ann Intensive Care 2019;9:82. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Harley O, Reynolds C, Nair P, et al. Long-Term Survival, Posttraumatic Stress, and Quality of Life post Extracorporeal Membrane Oxygenation. ASAIO J 2020;66:909-14. [Crossref] [PubMed]
Cite this article as: Biblowitz K, Mullin M, McDermott L, Sykuta A, Baram M, Hirose H. A multidisciplinary approach to prolonged extracorporeal membrane oxygenation for acute respiratory distress syndrome due to coronavirus 2019—case report. AME Case Rep 2022;6:8.


