Missing lung nodule? Intra-operative contingency plan with O-arm imaging: a case report
Introduction
Despite the availability of various modalities to locate small non-palpable pulmonary nodules during minimally invasive thoracoscopic surgery, precise lung nodule resection is still challenging (1). Pre-operative localization can be performed through various strategies, but time, expenses, and complications remain problematic (2). Intra-operative ultrasound is a real-time solution, but challenges remain with visualizing deep parenchyma lesions and operator-dependent use (3). Although true numbers are hard to find, many thoracoscopic wedge resections are performed using a combination of pre-operative imaging and intra-operative landmarks (4). While usually cost and time-efficient, the problem occurs when a wedge resection is performed, and the nodule is not within the specimen. Rather than proceeding to a more extensive resection, converting to a larger incision or imaging later, we present an additional option to help with intra-operative localization.
This case report describes the use of the O-arm Surgical Imaging System, which is used mainly by spine surgeons. The O-arm is a full-rotation imaging system that provides three-dimensional cone-beam imaging (5,6). It allows a standard operating room to function as a hybrid room when needed. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-21-71/rc).
Case presentation
We evaluated an 81-year-old male with a solid 8 mm left lower lobe (LLL) lung nodule, which had increased in size from 6 mm over the previous year. This nodule was detected during surveillance imaging for his previous stage 3 renal cell carcinoma (RCC) treated with a left nephrectomy 5 years prior to presentation. We suspected metastatic lung tumor of RCC. There was no other evidence of disease by imaging.
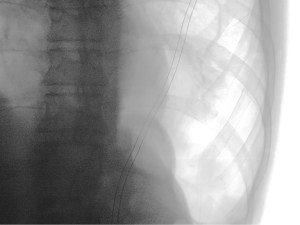
We brought the patient to the operating room for a diagnostic and therapeutic video-assisted thoracoscopic LLL wedge resection. We did not obtain pre-operative guidance other than CT scans in axial, coronal, and sagittal formatting (Figure 1). The nodule was 15 mm from the pleural surface. We placed the patient in the standard right lateral decubitus position. Two 5 mm and one 12 mm ports were placed in the lower aspect of the left chest. Despite carbon dioxide insufflation, we were unable to visually localize nor palpate the nodule with instruments. Using pre-operative imaging and intra-operative landmarks as a guide, we performed two consecutive wedge resections of the lateral-inferior aspect of the LLL. Unfortunately, we could not find the nodule in either specimen after back table gross examination. We were uncertain if the nodule had been resected and incorporated into a staple line or if we missed it. To avoid additional “blind” wedge resections or conversion to a thoracotomy, we used the O-arm to obtain intra-operative CT images.
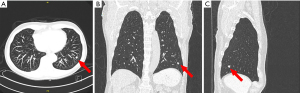
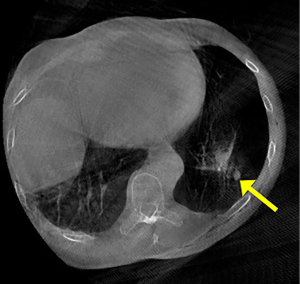
The patient remained sterile, in the lateral decubitus position, and we re-inflated the operative lung. We brought the O-arm over the patient’s chest and closed the ring. We centered the patient’s right thorax within the ring and approximated the position. We performed a fluoroscopic image to confirm the position over the left lower pleural space (Figure 2). We then imaged 16 cm in a cranio-caudal direction covering a majority of the LLL. The nodule was demonstrated adjacent to the staple line (Figure 3). Using the O-arm images and previous staple lines as a guide, we successfully resected the nodule with a third wedge resection.
Post-operatively, the patient did well and was discharged home two days later. Pathology reported a metastatic high-grade RCC with a 1.5 cm surgical margin.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Thoracoscopic localization of non-palpable nodules can be challenging. Risk factors for difficult localization include small nodules (<1 cm), nodules located >1 cm from the visceral surface, nodules located further from the visceral surface than the diameter of the nodule, and nodules located on an ovoid or convex portion of the lung (7,8). In one study, failure to locate pulmonary nodules during video-assisted thoracoscopic surgery (VATS) was the reason for conversion to thoracotomy in 23 (25%) out of 92 patients (9). Multiple techniques and technologies have been proposed to address this issue (10). Most techniques involve pre-operative marking of the nodule, but each technique adds additional time, expense, and complication rate (2,11). Real-time intraoperative localization through VATS-US would be an optimal solution, but challenges remain with visualization of deep (>2 cm) parenchyma lesions, ground-glass lesions, and visualization of nodules in patients with emphysema (11).
In contrast to this trend of pre-operative localization, other studies have shown that it is possible to resect non-palpable nodules without adjunct localization techniques. Using only pre-operative imaging and intra-operative landmarks, Kao successfully resected 26 (96.3%) of 27 nodules. All of the nodules were <2 cm and not palpable or detectable during VATS. Although actual numbers are hard to find, many thoracoscopic resections are still performed using a combination of pre-operative imaging and intra-operative landmarks (4). This approach streamlines care by avoiding pre-operative procedures. It is also cost-effective because it can be done in operating rooms with standard thoracoscopic equipment. The problem occurs when the resection is performed and the nodule is not within the specimen. The O-arm offers a back-up plan. It functions like a contingency hybrid operating room without the fixed costs of such a room. Importantly, it allows intra-operative CT imaging without repositioning the patient. Ohtaka et al. studied the O-arm as an intra-operative method for locating small lung nodules. Using pre-operative scans, they estimated the nodule’s location with a percutaneous needle and then used the O-arm to confirm the placement of the needle in relation to the nodule. If the needle was not accurately placed, it was repositioned, and a repeat scan was performed. Using this method, they successfully resected nodules, not on the visceral pleura, with a median size of 10 mm, from 9 out of 10 patients (5). As opposed to marker localization, ours is the first report of using the O-arm as a back-up plan for a missing nodule.
There are a few things to note when using the O-arm. Since the O-arm scans only 16 cm (7.2 inches) in length, the craniocaudal position must be estimated accurately. Also, the scanning window size is smaller. In other words, the area to be scanned (the left pleural space in our example) should be centered as much as possible within the ring of the O-arm (Figure 3). It (the O-arm) has a fluoroscopy function to help center the area to be imaged (Figure 2).
Additionally, like a C-arm, the patient may need to be repositioned on the table and the table must be C-arm compatible. Finally, the O-arm resolution is not as high as a 64 slice CT scanner. It has 7-line pairs per centimeter (lp/cm) versus 8 lp/cm for a 64 slice CT scanner. Nevertheless, it has high enough resolution to demonstrate an 8 mm lung nodule easily. On the plus side, the radiation dose delivered to the patient using the O-arm is half of the dose for a similar-sized using a 64 slice CT scanner (6).
Theoretically, relying on intraoperative landmarks may result in less accurate wedge resections i.e., smaller surgical margins compared to other localization techniques (4). However, this has not been proven in any prospective or even retrospective study. Any purported benefits to pre-operative localization must be weighed against the time, expense, and complications that such a technique entail. The technique described by Kao (4) requires experience and therefore is surgeon dependent. This debate regarding localization techniques does not detract from the O-arm system’s benefits when faced with a missing nodule. The O-arm Imaging System is a helpful technology that can be adapted from one surgical specialty to another.
Conclusions
The O-arm Imaging System functioned to convert a standard thoracoscopy room into a hybrid operating room. Rather than convert to a thoracotomy, proceed to a larger resection, or experience a missed nodule, the O-arm provided instant feedback regarding the status of a nodule. In this report, it was a helpful intra-operative tool to find a missing lung nodule.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-21-71/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-21-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sato T, Yutaka Y, Nakamura T, et al. First clinical application of radiofrequency identification (RFID) marking system-Precise localization of a small lung nodule. JTCVS Tech 2020;4:301-4. [Crossref] [PubMed]
- McDermott S, Fintelmann FJ, Bierhals AJ, et al. Image-guided Preoperative Localization of Pulmonary Nodules for Video-assisted and Robotically Assisted Surgery. Radiographics 2019;39:1264-79. [Crossref] [PubMed]
- Wada H, Anayama T, Hirohashi K, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg 2016;49:690-7. [Crossref] [PubMed]
- Kao MW. Intracorporeal direct measurement for localizing peripheral pulmonary nodules during thoracoscopy. J Thorac Dis 2019;11:4119-26. [Crossref] [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracoscopic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [Crossref] [PubMed]
- Zhang J, Weir V, Fajardo L, et al. Dosimetric characterization of a cone-beam O-arm imaging system. J Xray Sci Technol 2009;17:305-17. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Wright CD. Navigating the lung for hidden treasure. J Thorac Cardiovasc Surg 2018;156:1702-3. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Cornella KN, Repper DC, Palafox BA, et al. A Surgeon's Guide for Various Lung Nodule Localization Techniques and the Newest Technologies. Innovations (Phila) 2021;16:26-33. [Crossref] [PubMed]
- Eguchi T, Sato T, Shimizu K. Technical Advances in Segmentectomy for Lung Cancer: A Minimally Invasive Strategy for Deep, Small, and Impalpable Tumors. Cancers (Basel) 2021;13:3137. [Crossref] [PubMed]
Cite this article as: Castillo-Larios R, Hernandez-Rojas D, Paciotti B, Lee-Mateus AY, Pulipaka P, Fernandez-Bussy S, Makey IA. Missing lung nodule? Intra-operative contingency plan with O-arm imaging: a case report. AME Case Rep 2022;6:11.