
Porphyria cutanea tarda treated with short-term high-dose hydroxychloroquine: a case report
Introduction
Porphyria Cutanea Tarda (PCT) is classified as familial or sporadic forms and is mainly caused by an inherited or acquired deficiency of uroporphyrinogen decarboxylase, the fifth enzyme in heme synthesis, which catalyzes the decarboxylation of uroporphyrinogen into coproporphyrinogen (1). In addition to autosomal dominant inheritance, many exogenous factors can trigger the disease, such as hepatitis C, acquired immunodeficiency syndrome, alcoholism, sun exposure, and drug factors (2). The disease is manifested primarily in patients with elevated skin fragility and greater sensitivity to sunlight. Hypertrichosis and hyperpigmentation can occur in exposed areas such as the hands and face. Blisters that rupture with light touch may also occur repeatedly. The blisters may rupture and heal spontaneously to form a scar. This repeated injury can cause the skin to thicken and harden, eventually forming pseudosclerosis-like changes. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-21-77/rc).
Case presentation
A 64-year-old Chinese man was presented to the dermatology department with 1-year history of multiple easy-to-rupture blisters on the back of his hands. He reported recurrent multiple blisters for more than 1 year, which were aggravated by sun exposure. These blisters broke upon being touched but healed spontaneously to form scars. The patient was a farmer with no history of alcohol consumption and no similar cases in his family. He had no prior therapeutic intervention.
Physical examination showed brown pigmentation on the back of both hands along with scattered, isolated clear blisters, which were about 0.2×0.3 cm in size. These blisters broke upon being touched with a swab and exuded clarified herpes fluid. They were also accompanied by multiple skin breakdown crusts along with hard, light pink scars without any pain or itching. Moreover, brown pigmentation with hypertrichosis was seen on the face of the patient (Figure 1). We performed laboratory tests (Table 1) and dermatopathological biopsy on this patient.
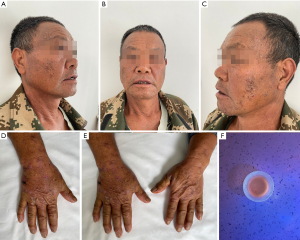
Table 1
| Test items | Normal | First | Second | Third |
|---|---|---|---|---|
| Urine porphyrin assay | Negative | Positive | – | – |
| Fecal porphyrin assay | Negative | Positive | – | – |
| AST (U/L) | 0–50 | 40 | 68 | 39 |
| ALT (U/L) | 0–50 | 57 | 114 | 51 |
| GGT (U/L) | 0–55 | 67 | 143 | 50 |
| ALP (U/L) | 40–150 | 89 | 152 | 86 |
| Ferritin (ng/mL) | 23–336 | 403 | 175 | 168 |
| Complete blood count, hepatitis C, syphilis, HIV, and tumor marker (AFP, CEA) | Negative | Negative | – | – |
| CT abdomen | Negative | Negative | – | – |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen.
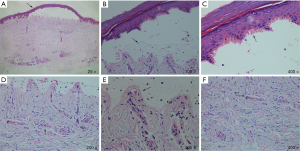
Dermatopathological biopsy showed the following results: the blister walls indicated microscopically intact subepidermal blisters with linearly arranged segmental homogeneous caterpillar bodies, similar to keratinized imperfect cells. A small number of inflammatory cells had infiltrated the blister while the intact dermal papillae protruded into the blister in the form of colored spheres. Sparse hyaline material was seen around the vessels of the dermal papillae, along with the change of gelatinous milia-like helioelastic tissue at the bottom of the blister (Figure 2).
The results of each test for the patient. Comparison of results includes comparison of test values and normal values, as well as comparison of initial test results and review results.
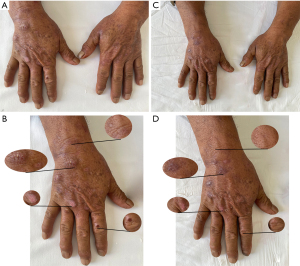
A diagnosis of PCT was made based on the patient’s medical history, typical skin lesion presentation, supportive laboratory tests, and pathological findings (3). Among them, The positive urine and fecal porphyrin tests in this patient played a key role in the diagnosis. Quantitative analysis of urinary porphyrins and serum porphyrins could not be performed due to lack of equipment. These two data are more helpful for our assessment of efficacy. The treatment regimen included 2×100 mg oral hydroxychloroquine (twice/day) to promote the metabolism of hepatic porphyrins and topical polymyxin ointment (0.5 g/time) to protect the wound surface and prevent infection. The patient was instructed to pay attention to sun protection, abstain from smoking and alcohol, and reduce the intake of food with high iron content. After 2 weeks of medication, the patient reported that he was feeling better and no new blisters were appearing. However, due to work, complete sun protection is not possible. According to the second laboratory tests results (Table 1). We found that his liver function was impaired. Thus, hydroxychloroquine was immediately discontinued, and to protect the liver, 2×100 mg of reduced glutathione tablets (3 times/day) were administered orally. Also, the topical compounded polymyxin ointment was added (twice/day). Two weeks later, the lesions on the hands resolved completely, and the liver function indicators returned to normal (Figure 3). Treatment with topical medication was continued.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In terms of treatment, the standard care for PCT involved periodic phlebotomy bloodletting (450 cc/dose) for reducing iron and porphyrin levels. Since phlebectomy is an invasive procedure requiring approximately 5–8 treatment sessions to achieve complete remission, the patient’s wishes and financial situation were taken into consideration, and a more moderate hydroxychloroquine treatment was finally chosen. The standard care for PCT was long-term oral administration of low-dose hydroxychloroquine (2×100 mg/week) to reduce the level of porphyrin in liver and blood (4). This patient had a higher dose of hydroxychloroquine, and the lesions regressed more rapidly. Although drug-related liver injury occurred, no new lesions appeared even after 2 weeks of discontinuation. The patient felt that the medication was very effective and that he no longer had new blisters on the back of his hands. He also did not experience any discomfort while taking the medication. In fect, this dose of hydroxychloroquine falls within the safe dose range. The patient showed an elevation in GGT that could be related to this drug, but we noted that the patient had abnormal GGT before taking hydroxychloroquine. Therefore, it cannot be excluded that hepatic impairment had already occurred prior to the administration of the drug. Thus, this suggests that an appropriate increase in the dose of hydroxychloroquine over a short period may be more effective than a small dose taken over a long time. However, the possibility of the patient having drug intolerance should also be considered. We should monitor further for changes in liver function during the drug administration, and upon intolerance, the drug should be discontinued immediately, and the patient should be provided with appropriate liver-protective drugs to promote the recovery of liver function.
Health education is especially important in these patients because there is a high number of predisposing factors causing this disease than genetic factors. Although the patient of ours had good daily habits and did not smoke or drink alcohol, the patient still needed to be taught how to perform proper and effective sun protection in daily life. To have alertness toward liver lesions, it is also important to conduct follow up with the patient for a long period.
In summary, although PCT is a rare liver disease with skin damage as a manifestation, it should be considered when there are recurrent painless blisters and scarring at the site of exposure. The diagnosis can be confirmed by testing for porphyrins in the urine, feces, or plasma. A thorough examination was also performed to rule out other comorbidities. Finally, short-term oral administration of higher doses of hydroxychloroquine could be preferred for the treatment regimen of PCT. However, liver function also needs to be monitored to prevent the development of drug-related liver injury. However, this treatment option needs to confirm by more research.
Acknowledgments
Funding: This work was supported by the Yunnan Provincial Department of Education Science Research Fund Project (No. 2021Y426).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-21-77/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-21-77/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-21-77/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bleasel NR, Varigos GA. Porphyria cutanea tarda. Australas J Dermatol 2000;41:197-206; quiz 207-8. [Crossref] [PubMed]
- Bygum A, Christiansen L, Petersen NE, et al. Familial and sporadic porphyria cutanea tarda: clinical, biochemical and genetic features with emphasis on iron status. Acta Derm Venereol 2003;83:115-20. [Crossref] [PubMed]
- Sassa S. Modern diagnosis and management of the porphyrias. Br J Haematol 2006;135:281-92. [Crossref] [PubMed]
- Singal AK, Kormos-Hallberg C, Lee C, et al. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin Gastroenterol Hepatol 2012;10:1402-9. [Crossref] [PubMed]
Cite this article as: Duan Y, Ni C, Huang L. Porphyria cutanea tarda treated with short-term high-dose hydroxychloroquine: a case report. AME Case Rep 2022;6:19.


