
Solid and papillary neoplasm of the pancreas (SPNP): a case report
Introduction
Solid and papillary neoplasm of the pancreas (SPNP), also known as a solid-cystic neoplasm, papillary cystic neoplasm, and Gruber-Frantz tumor, is a malignant tumor of the exocrine pancreas. Unlike most pancreatic exocrine neoplasms, however, it has a low malignancy grade and favorable prognosis. In 1996, the World Health Organization (WHO) classified this tumor as belonging to the international histological classification among neoplasms of the exocrine pancreas (1).
This tumor predominantly affects young women and presents as a voluminous abdominal mass. In most cases, it is asymptomatic and discovered by chance during routine examinations. Surgical treatment, even in the presence of distant metastases, is the only treatment guaranteeing a strong chance of survival. The role of neoadjuvant and/or adjuvant chemo-radiation therapy in the treatment of this tumor is not yet well-defined (2). As this tumor predominantly occurs in young women, this paper describes the case of a 34-year-old female presenting as having an incidental pancreatic mass confused as pancreatic adenocarcinoma. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-30/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
We present the case of a 34-year-old female without relevant familial history, who came to our hospital presenting with approximately a month of epigastric pain associated with dyspepsia, post-prandial abdominal distension, and uterine leiomyomatosis. During the evaluation, palpation and an abdominal ultrasound revealed a pancreatic growth that was “bulky, solid, with irregular margins, in homogeneously hypoechoic, with anechoic areas of necrosis, located lateral to the tail of the pancreas and medial to the upper pole of the left kidney and the lower splenic pole”. The patient’s history denied the use of estrogen-progestin drugs and abdominal trauma. Cancer markers were all normal [alpha fetoprotein (AFP) 1.3 ng/mL, carcinoembryonic antigen (CEA) <0.50 ng/mL, tissue polypeptide-specific antigen (TPS) 38.8 ng/mL, carbohydrate antigen (CA) 19-9 <2 U/mL, CA 125 4.5 U/mL, CA 15-3 15.8 U/mL], as well as routine blood chemistry tests except for a modest increase in alkaline phosphatase (ALP) (141 U/L), alanine-glyoxylate aminotransferase (AGT) (70 U/L) and lactate dehydrogenase (LDH) (614 U/L); the blood count showed neutrophilic leukocytosis (12.36×103/mL) and normal platelets (720×103/mL).
The patient also had a percutaneous guided core-needle biopsy (at our institution), which led to a diagnosis of adenocarcinoma (stage 2). The patient was admitted, and surgery was performed. At the laparotomy, with a left subcostal incision widened to the right, a tumor of 15 cm in diameter was detected. The tumor was located in the tail of the pancreas, was well encapsulated, and of solid consistency. Caudal pancreatectomy with a splenectomy was carried out. There was no metastatic disease. The postoperative course was good, and the patient was discharged on the seventh day post-operative in a good general condition.
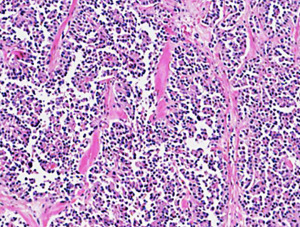
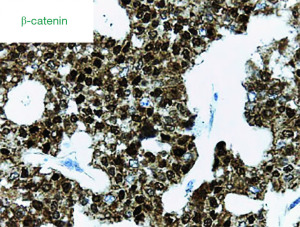
The surgical specimen measured 15 cm × 13 cm × 9 cm and macroscopically appeared to be completely occupied by a necrotic and hemorrhagic mass. Extensive sampling in the peripheral areas revealed a tumor characterized by solid cell nests with richly vascularized fibrous stroma; sometimes a trabecular pattern with hyaline or myxoid stroma; a pattern that became pseudopapillary, with a small central vessel; and, finally, pseudo rosettes, where there was a loss of cohesion (Figure 1). The capsule was locally infiltrated by groups of tumor cells. The immunophenotype was negative for chromogranin, cytokeratin (AE1/AE3), CD10, and it was diffusely positive for vimentin, synaptophysin, progesterone, alpha-1-antitrypsin, and CD56. The neoplasm was weakly positive for neuron-specific enolase (NSE) and showed strong nuclear positivity for β-catenin (Figure 2). The definitive histological diagnosis was an SPNP with a capsular invasion and tumor-free resection margin.
At 12 months post-surgery, the patient is in good clinical health, including in a specific oncological follow-up program.
Discussion
The SPNP represents approximately 1–2% of all pancreatic tumors (3). In the past, it was often confused with acinar cell carcinoma or a cystic variant of pancreatic tumors, but in the last 10 years, there has been an increase in its incidence, likely due to greater knowledge of it. It is known to predominantly affect young women between the second and third decade of life; however, the literature describes a case of a very young patient of just two years and the eldest reported patient at 81 years (4). The SPNP is exceptional in the male sex, and its prevalence is greater in the African-American and Asian population (5). The tumor can be localized in any segment of the pancreas, but from a review of the literature, it was found to involve the tail in 36% of cases, the head in 34% of cases, and could be extra pancreatic in 1% of cases (6). Among the numerous hypotheses made on the etiopathogenesis of this neoplasm, the most credible one seems to be based on increased production of ovarian hormones during development (7), both for the almost absolute prevalence in young women and because of is described positivity to progesterone receptors in 90% of SPNP (8). Current knowledge of the molecular biology of SPNP is limited, but CTNNB1 gene mutations, a gene that encodes β-catenin and is located on chromosome 3p, have been reported in over 90% of SPN tumors (9). Mutations that are commonly seen in pancreatic ductal adenocarcinoma, such as KRAS, P53, P16, and DPC4, have not been seen with SPNP. A limitation of our case is the unavailability of genetic tests.
Clinical symptoms are nonspecific—and many patients are often even asymptomatic. It presents as a large abdominal swelling, associated with mild pain, dyspepsia, sometimes jaundice, intestinal obstruction, hemoperitoneum from capsular rupture, and more rarely with pancreatitis (10). Normally, laboratory tests (e.g., those testing for pancreatic oncological markers, serum, and urinary levels of amylase and glycaemia) do not show any significant variation. A correct pre-operative diagnosis is fundamental given the low malignancy grade and the good prognosis after radical removal. Some authors recommend performing an ultrasound-guided fine-needle biopsy for pre-operative diagnosis (11), which is in total contrast to others, who underline the high number of false negatives and the potential risk of spreading cancer cells using this method (12).
Indispensable for the purposes of a correct diagnostic classification is the histopathological examination, with a histology characterized by solid and cystic structures, often alternating with areas of hemorrhagic infarction and necrosis and cells in a papillary formation, with a very low number of mitoses. The main differential histopathology diagnosis is with ductal carcinomas, mucinous neoplasms, serous neoplasm, acinar neoplasm, and neuroendocrine tumors. Generally, the pathology diagnosis is straightforward, but in cases with limited samples and cases with a predominance of necrosis and nuclear pleomorphism, the diagnosis can be quite challenging. In these cases, immunohistochemistry is very useful. The SPNP shows negativity to immunohistochemistry for chromogranin, trypsin and chymotrypsin (results that exclude endocrine and acinar tumors); it is positive for vimentin, progesterone, CD56, and alpha-1-antitrypsin, as well as a frequent mutation in the gene that codes for β-catenin (13). Focal positivity for NSE is sometimes present (14). CD10 is reported to be positive in nearly 100% of the cases, but scarce and negative immunoreaction has been described (15), especially in necrotic tumors. Macroscopically, the tumor tissue is well-demarcated from normal pancreatic tissue by the presence of a thick fibrous capsule. In 85% of cases, at the time of diagnosis, the tumor is limited to the pancreas, and in the remaining 15% of cases, the metastatic involvement predominantly concerns the liver and peritoneum, and lymph-node involvement is exceptionally rare (16).
From a surgical point of view, an aggressive approach is justified, even in the presence of hepatic and/or peritoneal metastases. This is so by the “favorable” biological behavior of the tumor (low-grade malignancy, slow growth, low tendency to vascular invasion) and by the fact that complete surgical excision is curative in 95% of cases of SPNP localized to the pancreas. Notwithstanding the oncological principles valid for most malignant tumors of the pancreas, for the treatment of metastases, there is a common consensus to perform surgical de-bulking for the good results described in the literature in terms of long-term survival (17). Since in most cases the tumor is well-delimited by a thick fibrous capsule, it is possible to carry out conservative resections while sparing normal pancreatic tissue. Depending on the location of the tumor, surgical interventions can range from simple resection to corpus caudal pancreatectomy, with or without spleen preserving, to cephalic pancreatoduodenectomy. Performing any type of lymphadenectomy is unnecessary.
Given the rarity of this neoplasm, precise prognostic factors have not yet been identified. Vascular invasion, perineural invasion, nuclear pleomorphism, involvement of the surrounding pancreatic parenchyma, as well as the presence of distant metastases seem predictive of aggressive behavior. The role of neoadjuvant and/or adjuvant chemo-radiotherapy in the treatment of SPNP is not yet well-defined, which is also due to the high percentage of remission in cases involving this tumor.
Conclusions
In the presence of a large abdominal mass of pancreatic relevance, even in older women, the possibility of having an SPNP should always be evaluated. Given the low malignancy potential of this tumor and the excellent prognosis with radical surgical treatment, the preoperative diagnosis should always be particularly accurate. Surgical resection is recommended as the treatment of choice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-30/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-30/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frantz VK. Tumors of the pancreas. In the Atlas of tumor Pathology. Washington DC: Armed Forces Institute of Pathology, 1959:32-3.
- Zhang H, Liang TB, Wang WL, et al. Diagnosis and treatment of solid-pseudopapillary tumor of the pancreas. Hepatobiliary Pancreat Dis Int 2006;5:454-8. [PubMed]
- Washington K. Solid-pseudopapillary tumor of the pancreas: challenges presented by an unusual pancreatic neoplasm. Ann Surg Oncol 2002;9:3-4. [Crossref] [PubMed]
- Wang P, Wei J, Wu J, et al. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas: A single institution experience with 97 cases. Pancreatology 2018;18:415-9. [Crossref] [PubMed]
- Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg 1999;23:1045-50. [Crossref] [PubMed]
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965-72. [Crossref] [PubMed]
- Tipton SG, Smyrk TC, Sarr MG, et al. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg 2006;93:733-7. [Crossref] [PubMed]
- Kosmahl M, Seada LS, Jänig U, et al. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch 2000;436:473-80. [Crossref] [PubMed]
- Kim S, Jeong S. Mutation Hotspots in the β-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol Cells 2019;42:8-16. [PubMed]
- Omori H, Asahi H, Inoue Y, et al. Solid and cystic tumor of the pancreas with massive hemoperitoneum. Hepatogastroenterology 2005;52:936-9. [PubMed]
- Bardales RH, Centeno B, Mallery JS, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol 2004;121:654-62. [Crossref] [PubMed]
- Raffel A, Cupisti K, Krausch M, et al. Therapeutic strategy of papillary cystic and solid neoplasm (PCSN): a rare non-endocrine tumor of the pancreas in children. Surg Oncol 2004;13:1-6. [Crossref] [PubMed]
- Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol 2002;160:1361-9. [Crossref] [PubMed]
- Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP 2006;7:131-6. [PubMed]
- Notohara K, Hamazaki S, Tsukayama C, et al. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol 2000;24:1361-71. [Crossref] [PubMed]
- Matsunou H, Konishi F, Yamamichi N, et al. Solid, infiltrating variety of papillary cystic neoplasm of the pancreas. Cancer 1990;65:2747-57. [Crossref] [PubMed]
- Ogawa T, Isaji S, Okamura K, et al. A case of radical resection for solid cystic tumor of the pancreas with widespread metastases in the liver and greater omentum. Am J Gastroenterol 1993;88:1436-9. [PubMed]
Cite this article as: Frías-Fernández P, Sánchez-Flores S, García-Chávez JP, Padilla-Rosciano AE, Lino-Silva LS. Solid and papillary neoplasm of the pancreas (SPNP): a case report. AME Case Rep 2022;6:38.


