Delayed metastatic pulmonary epithelioid hemangioendothelioma of the femoral vessels: case report and literature review
Highlight box
Key findings
• Indolent metastasis can occur in epithelioid hemangioendothelioma (EHE), despite a prolonged disease-free interval.
What is known and what is new?
• EHE is a rare sarcoma of the blood vessels.
• There are no previously known cases of indolent EHE with delayed metastases.
What is the implication, and what should change now?
• Long-term surveillance with serial imaging beyond 5 years may be beneficial.
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare sarcoma of the blood vessels, usually presenting with a poor prognosis or an indolent course. We report a study of a patient with delayed vascular EHE with pulmonary metastasis, of which there are no previously known reports. We represent the following article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-57/rc).
Case presentation
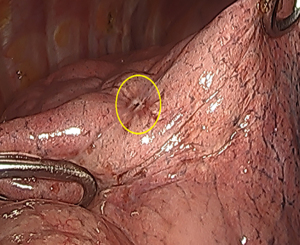
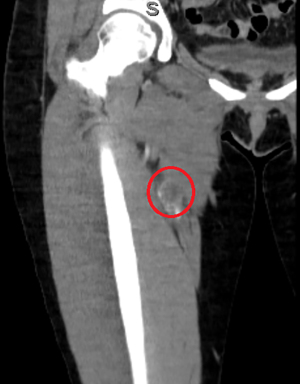
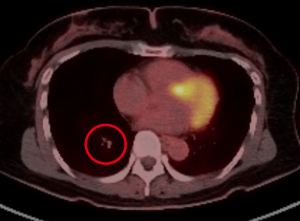
The patient is a 40-year-old female who presented with a painful right groin mass and lower extremity swelling. A duplex ultrasound showed deep vein thrombosis (DVT) of the femoral vein as well as a 3.8 cm mass in the femoral sheath; a computerized tomography (CT) scan also confirmed a soft tissue mass in the femoral sheath with an abutment of the femoral artery (Figure 1). The patient was started on warfarin for her DVT, resulting in improved edema, and was taken to surgery for resection of the mass, along with a groin exploration and resection of a segment of the femoral vein. The femoral artery was not involved grossly and therefore dissected off the mass. An intraoperative frozen section of the mass was inconclusive as it only showed myxoid tissue without any evidence of malignancy. However, the final pathology confirmed EHE based on histology and immunohistochemical (IHC) staining. A second surgery, involving a complete resection of the skin and soft tissue, as well as a segment of the femoral artery was subsequently performed because the initial margins were microscopically positive. Reconstruction of the femoral artery with a reverse greater saphenous vein bypass followed thereafter. The initial frozen section and ensuing final pathology showed a small focus of EHE with clear radial margins. The patient had an uneventful recovery followed by annual vascular duplex surveillance of the bypass graft. However, 12 years after the initial treatment for her right leg EHE, a surveillance chest X-ray identified a 7 mm nodule in the right lower lobe, confirmed by a positron emission tomography (PET) scan (Figure 2). A video-assisted thoracic surgery was performed with a wedge resection on the nodule (Figure 3), pathologically confirmed to be EHE with clear surgical margins. The patient remains disease-free after routine surveillance at the one-year mark.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
EHE is a rare sarcoma of the blood vessels. EHE was first described in 1975 by Dail and Liebow as a bronchioloalveolar tumor due to invasion of adjacent blood vessels and airways to the lungs; it was later termed in 1982 by Weiss and Enzinger as a separate tumor with characteristics between hemangiomas and angiosarcomas (1,2). The prevalence of the tumor is around 1/1,000,000 and mostly affects middle-aged individuals, with a slight prevalence in women. EHE is rare in children and the elderly (3). Our patient initially presented with a vascular EHE in the femoral vein; EHE can originate from any small or medium size blood vessel, and the most affected areas include the lung, liver, soft tissue, and bone. Larger vascular structures are less commonly affected, yet cases involving the aorta or vena cava have been reported (4-7). While patients are usually asymptomatic, some present with tumor-related symptoms such as pain, weight loss, fatigue, anemia, and a noticeable mass (3). Severe respiratory symptoms (cough, dyspnea), pleural hemorrhagic effusion, multiorgan involvement, and metastases have been correlated with a worse prognosis in patients with pleural and/or pulmonary EHE (3,8).
Due to its rarity and similarities to other sarcomas, EHE is difficult to effectively diagnose without a full pathological review. EHE’s histological findings appear as spindle-shaped cells or abnormal cell arrangements (complex branching, pseudopapillary, pseudo glandular) (1). Confirmatory IHC stains are positive for CD31, CD34, FLI-1, and Factor VIII but are negative for AE1/AE3 markers (3). Chromosome translocations resulting in WWTR1-CAMTA1 or YAP1-TFE3 gene fusions have been identified (3). EHE is also hypothesized to be correlated with chronic Bartonella infection, as the bacteria prevents endothelial cell regulation, which hinders apoptosis and contributes to tumor growth (1). Our patient’s IHCs were positive for CD34 and CD31, and negative for AE1/AE3 and S100 protein; low mitotic activity was observed with less than 1/50 per high power field.
EHE tends to have low to moderate activity on 18F-fluorodeoxyglucose (FDG)-PET/CT scans, although high uptake has been reported with aggressive pulmonary EHE (9,10). PET scan may help detect occult disease in the liver or lung (10).
Pulmonary EHE usually presents as bilateral small nodules in patients rather than single lesions, with a chance to spread to extrapulmonary regions. Multiple nodules could be a result of malignant neoplasms or multiple primary origins (11). Pulmonary EHE can easily be misdiagnosed as other lung diseases and a lung biopsy is used to make a diagnosis. The 5-year survival varies from 73% to 96.3% overall in patients with pulmonary EHE, with patients developing pleural effusion or extra-pleural disease having a worse prognosis (11,12). The prognosis is favorable for patients with slow disease progression and without metastatic disease outside the lung or bone involvement (13).
There is no standard treatment for EHE; it is unique on a patient-to-patient basis, often resulting in suboptimal outcomes. Complete surgical resection remains the main form of treatment to achieve negative margins and minimize the chance of recurrence, especially when the sarcoma presents as a single mass. Our patient had one solitary activity in the right lung, so a surgical resection with curative intent was performed. Surgery can be coupled with chemotherapy and/or radiation in cases with unresectable tumors, although it has not been proven effective in most cases (3,4). Likewise, debulking surgeries and radiofrequency ablations (RFA) are options when the tumor has spread beyond its original site (4). Systemic treatment is not supported with resectable tumors, especially given their slow and unpredictable nature (3,4). Patients with metastatic disease are sometimes given anti-tumor drugs which slow down the progression of the disease by acting as inhibitors with specific protein targets such as mechanistic target of rapamycin (mTOR) or mitogen-activated protein kinase (MEK) inhibitors. Such inhibitors are currently under investigation for advanced non-resectable EHE. After treatment, patients should continue to be monitored to observe the progression of the disease (3).
According to various authors, metastases can occur even before the primary diagnosis and cells can remain dormant for a prolonged period. The angiogenic and immunologic models explain dormancy as a reliance of the tumor cells on the vascular system and the resulting balance between proliferation and apoptosis through the activity of the immune system. Alternatively, the cellular dormancy model describes the role of cyclin-dependent kinases (CDK), transcription factors, and downregulation of signaling cascades in causing cells to enter a stage of cell-cycle arrest (quiescence) (14). Such metastases are not detected until cells come out of the latent period and tend toward the growth of the tumor (14,15). A patient with a liver transplant due to hepatic EHE, observed in a case study by Tan et al., succumbed to the disease following delayed metastasis (16).
Conclusions
Our patient most likely had delayed metastasis of EHE to the lung, 12 years after the initial treatment. However, a second, unrelated case of EHE cannot be completely ruled out. While the prognosis for our patient is favorable given the latent solitary lesion without evidence of metastatic disease in the bone or extra-pleural tissue, continued surveillance with serial images will be necessary. Long-term surveillance beyond 5 years may be beneficial due to the delayed metastasis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-57/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-57/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-57/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;8:259. [Crossref] [PubMed]
- Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970-81. [Crossref] [PubMed]
- Stacchiotti S, Miah AB, Frezza AM, et al. Epithelioid hemangioendothelioma, an ultra-rare cancer: a consensus paper from the community of experts. ESMO Open 2021;6:100170. [Crossref] [PubMed]
- Witte S, Weidema M, Kaal S, et al. The heterogeneity of Epithelioid Hemangioendothelioma (EHE): A case series and review of the literature with emphasis on treatment options. Semin Oncol 2021;48:111-8. [Crossref] [PubMed]
- Minyi Y, Xintian H, Weimin L, et al. Intravascular epithelioid hemangioendothelioma. Int Angiol 2011;30:181-4.
- Alam SI, Nepal P, Sajid S, et al. Epithelioid Hemangioendothelioma of the Ulnar Artery Presenting with Neuropathy. Ann Vasc Surg 2020;67:563.e13-7. [Crossref] [PubMed]
- Elliott IA, Kasinpila P, Guenthart BA, et al. Resection of a Giant Epithelioid Hemangioendothelioma Arising from the Superior Vena Cava. Ann Thorac Surg 2021;112:e257-60. [Crossref] [PubMed]
- Bagan P, Hassan M, Le Pimpec Barthes F, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg 2006;82:2010-3. [Crossref] [PubMed]
- Watanabe S, Yano F, Kita T, et al. 18F-FDG-PET/CT as an indicator for resection of pulmonary epithelioid hemangioendothelioma. Ann Nucl Med 2008;22:521-4. [Crossref] [PubMed]
- Wei W, Huang G, Liu J. Advancing the diagnosis of epithelioid hemangioendothelioma by (18)F-FDG PET/CT. Am J Nucl Med Mol Imaging 2021;11:230-2.
- Onishi Y, Kusumoto M, Goto Y, et al. Epithelioid hemangioendothelioma of the lung: CT findings and clinical course of 35 cases. Jpn J Clin Oncol 2020;50:1195-200. [Crossref] [PubMed]
- Kitaichi M, Nagai S, Nishimura K, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J 1998;12:89-96. [Crossref] [PubMed]
- Mesquita RD, Sousa M, Trinidad C, et al. New Insights about Pulmonary Epithelioid Hemangioendothelioma: Review of the Literature and Two Case Reports. Case Rep Radiol 2017;2017:5972940. [Crossref] [PubMed]
- Yeh AC, Ramaswamy S. Mechanisms of Cancer Cell Dormancy--Another Hallmark of Cancer? Cancer Res 2015;75:5014-22. [Crossref] [PubMed]
- Friberg S, Nyström A. Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis 2015;8:43-9. [Crossref] [PubMed]
- Tan Y, Yang X, Dong C, et al. Diffuse hepatic epithelioid hemangioendothelioma with multiple splenic metastasis and delayed multifocal bone metastasis after liver transplantation on FDG PET/CT images: A case report. Medicine (Baltimore) 2018;97:e10728. [Crossref] [PubMed]
Cite this article as: Jirasirinuphan P, Chang AL, Deepak A, Chang CK. Delayed metastatic pulmonary epithelioid hemangioendothelioma of the femoral vessels: case report and literature review. AME Case Rep 2023;7:1.