
The relationship between PARP inhibitors with the relapse and leukemisation of lymphomas: a case report
Highlight box
Key findings
• Possible increased of risk of lymphoma development with iPARP treatment.
What is known and what is new?
• The association between the risk of developing MDS or leukaemias with iPARP treatment is already known and described
• The increased risk of lymphoma development with iPARP is unknown.
What is the implication, and what should change now?
• Given the alterations in haematopoiesis caused by iPARPs and the possible increased risk of lymphoma development, if these findings are confirmed, closer monitoring of these patients would be necessary.
Introduction
The treatment of tumours with homologous recombination deficit has undergone a radical change in recent years. The introduction of the treatment of ovarian carcinomas using poly-ADP ribose polymerase inhibitor (iPARP) has led to increased survival and response rates in these tumours (1). Different clinical trials, such as SOLO-1 and PRIMA, have shown that progression-free survival (PFS) and disease control rates are much higher in those patients receiving maintenance therapy with iPARPs after platinum response (2,3). Although tolerance to these drugs is usually good, haematological toxicity is one of their main limitations. The occurrence of acute leukaemias and myelodysplastic syndromes (MDS) secondarily to iPARPs is known and has been identified in 0.5–1% of patients (4,5); however, there are no data on, nor associations with, the appearance or alteration of the course of the lymphomas described. In this report, we aim to assess the association of iPARPs with the development of lymphomas through the clinical case of a patient who suffered a relapse and transformation of leukaemia from a lymphoma in complete response after treatment with iPARP for ovarian carcinoma. We present the following article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-91/rc).
Case presentation
We present the case of a 78-year-old woman with a diagnosis of serous carcinoma of the ovary who was undergoing maintenance treatment with Niraparib. The patient was diagnosed in 2011 with stage IIIC serous ovarian carcinoma (unmutated somatic BRCA). After receiving a neoadjuvant with Paclitaxel-Carboplatin, the patient underwent surgery with R0 resection. Subsequently, she suffered two tumour recurrences at the pelvic level, in 2014 and 2019, both of which were treated with surgery (both R0 interventions) plus adjuvant chemotherapy. In the second of them, after a complete response, maintenance treatment with Niraparib was prescribed. It began in January 2020. In 2016, during follow-up on the second relapse, in 2014, the patient was diagnosed with diffuse large cell B lymphoma (DLBCL). She received treatment with an R-CHOP scheme for six cycles with complete response without recurrence or incidents in subsequent follow-up.
In March 2021, the patient sought treatment for a clinical evaluation of constitutional syndrome with abdominal pain. After performing the physical examination, during which no alterations were observed, an urgent blood test was conducted, and the presence of a bicytopenia (platelets 28,000/mcL, haemoglobin 9.6 g/dL) of unknown origin with an acute deterioration of renal function of prerenal origin was observed (Table 1). An urgent abdominal CT scan was requested, and a peritoneal carcinomatosis with free fluid secondary to ovarian tumour recurrence was observed. It was decided to admit the patient for transfusion support and a study of the bicytopenia. Erythroblastosis was observed in the requested blood smear, with signs compatible with bone marrow infiltration. After that, a bone marrow biopsy was performed. The anatomo-pathological results were compatible with bone marrow infiltration by high-grade B lymphoma (Figure 1), no other lesions compatible with medullary involvement by ovarian carcinoma were observed.
Table 1
| Determination | Results | Laboratory range |
|---|---|---|
| Glucose | 121 (mg/dL) | 76–110 (mg/dL) |
| Creatinine | 2.49 (mg/dL) | 0.5–0.9 (mg/dL) |
| Calcium | 9.6 (mg/dL) | 8.8–10.2 (mg/dL) |
| Sodium | 134 (mmol/L) | 135–145 (mmol/L) |
| Potassium | 4.4 (mmol/L) | 3.5–5.1 (mmol/L) |
| Total bilirubin | 0.45 (mg/dL) | 0.15–1.2 (mg/dL) |
| C-reactive protein | 3.19 (mg/dL) | 0–0.5 (mg/dL) |
| Haemoglobin | 9.6 (g/dL) | 12–16 (g/dL) |
| Leukocytes | 14.490 (/mcL) | 4.5–10.8 (/mcL) |
| Neutrophil | 9.250 (/mcL) | 1.4–6.5 (/mcL) |
| Platelets | 28.000 (/mcL) | 150–450 (/mcL) |
| pH | 7.34 | 7.35–7.45 |
| Lactate | 4.8 (mmol/L) | 0.5–1.6 (mmol/L) |
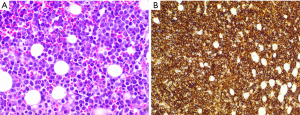
After the previous diagnosis, the patient developed an infection without focus (negative cultures and imaging tests), leading to the need for greater transfusion support in the forms of both blood and platelets. Treatment with piperacillin-tazobactam plus teicoplanin was initiated, and an evacuatory paracentesis was performed to improve renal function with diuretic treatment. Despite the measures in place, the patient died prior to the start of treatment for the lymphoid tumour.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The association between acute leukaemia and MDS with iPARPs is described in virtually all cases of ovarian carcinomas. The theoretical mechanism proposed for this relationship is derived from the possible action of iPARPs on the selection of acquired mutations in clonal haematopoiesis in the DNA damage response pathway (6). The risk factors that predict the possible development of these haematological alterations are not known; however, there are several hypotheses that relate the germinal mutations of BRCA1/2, TP53 or PALB2 with an increased risk of developing acute leukaemia or MDS (7,8).
In the case of our patient, there was a relapse with laeukemisation of DLBCL five years after the complete response to the R-CHOP scheme. The possibility of the existence of acquired mutations in pathological lymphoid cells that will lead to an increase in the probability of recurrence and greater aggressiveness of DLBCL is key. Treatment with Niraparib resulted in a 14-month PFS with no grade 3–4 toxicity. This is why the long exposure to iPARPs in this patient led to an increase in inadequate clonal haematopoiesis and the consequent lymphoid tumour recurrence (9). The early detection of cases at risk for the development of haematological alterations with iPARPs would allow a premature stopping of treatment with iPARP, or a different action in the follow-up protocols, for these patients. In addition to the iPARP treatment, the patient in this case had previously received multiple chemotherapy treatments (R-CHOP and Paclitaxel-Carboplatin), which may have led to an increase in haematopoiesis alterations, increasing the likelihood of recurrence of the haematological malignancy together with the iPARPs.
Although iPARP treatment has been associated with the development of MDS and leukaemias, its association with lymphomas is less clear. However, there are data supporting the association in the case of our patient. In the real-world data study by Zhao et al. (10), the latency period between the onset of iPARP and the onset or recurrence of haematological malignancy is like our case. Furthermore, a large percentage of cases presented as highly aggressive neoplasms with a high rate of fatal events. Therefore, although the association is novel, it is highly similar to cases of MDS and leukaemia.
Conclusions
In conclusion, the association of treatment with iPARP and the development of lymphomas is key for increasing knowledge of the safety profiles these drugs. The search for risk factors associated with the development of haematological neoplasms secondary to iPARP is essential for the future prevention of these pathologies in cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-91/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-91/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirza MR, Coleman RL, González-Martín A, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol 2020;31:1148-59. [Crossref] [PubMed]
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018;379:2495-505. [Crossref] [PubMed]
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381:2391-402. [Crossref] [PubMed]
- Morice PM, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 2021;8:e122-34. [Crossref] [PubMed]
- Tinker AV. PARP inhibitors-understanding the risk of myelodysplastic syndrome and acute myeloid leukaemia. Lancet Haematol 2021;8:e97-9. [Crossref] [PubMed]
- Bolton KL, Moukarzel LA, Ptashkin R, et al. The impact of poly ADP ribose polymerase (PARP) inhibitors on clonal hematopoiesis. J Clin Oncol 2020;38:abstr 1513.
- McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer 2017;17:513-27. [Crossref] [PubMed]
- Churpek JE, Marquez R, Neistadt B, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer 2016;122:304-11. [Crossref] [PubMed]
- Morton LM, Dores GM, Schonfeld SJ, et al. Association of Chemotherapy for Solid Tumors With Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA Oncol 2019;5:318-25. [Crossref] [PubMed]
- Zhao Q, Ma P, Fu P, et al. Myelodysplastic Syndrome/Acute Myeloid Leukemia Following the Use of Poly-ADP Ribose Polymerase (PARP) Inhibitors: A Real-World Analysis of Postmarketing Surveillance Data. Front Pharmacol 2022;13:912256. [Crossref] [PubMed]
Cite this article as: Navalón-Jiménez M, Olivares-Hernández A, Figuero-Pérez L, Miramontes-González JP, Montero-Mateos E, Cruz-Hernández JJ, Fonseca-Sánchez E. The relationship between PARP inhibitors with the relapse and leukemisation of lymphomas: a case report. AME Case Rep 2023;7:14.


