
Double orifice mitral valve: a report of two cases with different severity and treatment
Highlight box
Key findings
• The double orifice mitral valve (DOMV) can present with different severity ranging from asymptomatic to severe valvular dysfunction including mitral regurgitation (MR) and mitral stenosis, as well as, symptoms of coexisting congenital anomalies.
What is known and what is new?
• DOMV, a rare congenital mitral valve (MV) disease, usually coexists with other congenital heart diseases. It is characterized by a 2-orifice MV separated by a tissue bridge, causing a spectacles-like morphology.
• DOMV can be incidentally diagnosed during the investigation of various cardiovascular symptoms. It should be clearly identified whether the symptom is related to DOMV or not.
What is the implication, and what should change now?
• The treatment strategy for DOMV, e.g., surgery or watchful waiting, should be carefully considered based on the severity of patient presentations.
Introduction
Double orifice mitral valve (DOMV), a rare congenital mitral valve (MV) disease, usually coexists with other congenital heart diseases. It is characterized by a 2-orifice MV separated by a tissue bridge, causing a spectacles-like morphology. Patients with DOMV can be asymptomatic or present with symptoms of valvular dysfunction and concomitant congenital anomalies (e.g., atrioventricular canal defect, atrial septal defect, ventricular septal defect, patent ductus arteriosus, bicuspid aortic valve, etc.) (1). Echocardiography is the mainstay of investigation, while advanced multimodality imaging can provide additional information in selected patients. The surgical intervention is indicated when the patient develops symptomatic valvular dysfunction or a significant coexisting anomaly, while the conservative strategy is appropriate for asymptomatic patients. We present the following cases in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-52/rc).
Case presentation
Case 1
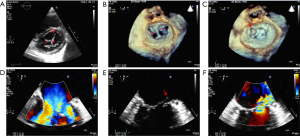
A 52-year-old woman was referred to our hospital due to progressive dyspnea with orthopnea for four months. She had no pertinent medical history. Physical examination revealed that the apical heartbeat shifted downward to the 6th intercostal space and leftward to the anterior axillary line, reflecting cardiomegaly. There was a grade III/VI holosystolic murmur at the cardiac apex radiating to the axilla. She also had a fine crackle in both lungs. A 12-lead electrocardiogram (ECG) showed the voltage criteria for left ventricular hypertrophy. Chest radiograph demonstrated an increased cardiothoracic ratio with a sign of pulmonary vascular cephalization. The transthoracic and transesophageal echocardiogram depicted DOMV with a severe posteriorly directed jet mitral regurgitation (MR) resulting from the ruptured chordae tendineae of the lateral scallop of the anterior mitral valve leaflet (AMVL) (Figure 1A-1F). There was moderate tricuspid regurgitation (TR) with an estimated right ventricular systolic pressure of 85 mmHg, indicating pulmonary hypertension.
Because of symptomatic severe MR and moderate TR, MV replacement with a 33-mm St. Jude Medical mechanical valve and tricuspid valve annuloplasty with a 28-mm Edwards annuloplasty ring were performed. Intraoperative findings confirmed an adhesion between AMVL and the posterior leaflet of MV with fibrous tissue forming 2-orifice MV together with thickening and the lateral scallop of AMVL. The perioperative period was uneventful. At the 3-month follow-up visit, she could perform routine daily activities and light exercises without symptoms of heart failure. A postoperative echocardiogram showed the normal functioning prosthetic MV without residual MR.
Case 2
A 36-year-old previously healthy woman presented with recurrent palpitation described by a skipped beat for one year. Her physical findings were unremarkable. A 12-lead ECG showed sinus rhythm with bigeminy premature ventricular contraction (PVC) originating from the right ventricular outflow tract (RVOT) area. A 24-hour Holter monitoring revealed frequent premature ventricular beats of more than 30,000 beats per day (35% burden). Subsequent transthoracic echocardiography was performed to evaluate left and right ventricular function as well as an associated structural heart condition. The study unexpectedly revealed a complete bridge-type DOMV without concomitant mitral stenosis (MS) nor MR (Figure 2A-2D). Other cardiac structures and functions were normal, including biventricular size and function. A cardiac magnetic resonance (CMR) imaging was scheduled to further evaluate the possible arrhythmogenic foci at RVOT. The CMR showed normal morphology of RVOT without scar or aneurysm. The phase-contrast image across the MV orifice nicely demonstrated a 2-orifice MV separated by a tissue bridge at the annulus, confirming the DOMV (Figure 2E,2F). No other associated cardiac anomaly was detected.

After discussing with the patient, she decided to treat an arrhythmia with medication; however, a beta-blocker did not adequately control her symptoms. Radiofrequency ablation was eventually performed with a satisfactory result. She reported no palpitation during the 6-month follow-up period. Given that there was no respective hemodynamic abnormality related to DOMV, the patient was managed conservatively treated with a regular evaluation of symptoms, physical examination, and repeat echocardiography when clinically indicated.
Consent and ethics
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
A DOMV is a rare MV abnormality with a prevalence of 0.05% as an isolated disease and 1% when it coexists with other congenital heart diseases (1,2). A DOMV is characterized by a 2-orifice MV separated by a tissue bridge at the annulus or leaflet level, causing a spectacles-like morphology. Each orifice has a separate subvalvular apparatus (chordae tendineae and papillary muscles) (3). There are two well-known classifications for congenital DOMV. First, echocardiogram-based classification by tilting ultrasound probe through the annular, midleaflet, and leaflet edge planes of the MV categorizes the DOMV into a complete bridge type (DOMV is seen in all planes), incomplete bridge type (DOMV is seen only in leaflet edge plane) and hole type (accessory orifice was seen in midleaflet plane) (3). The second classification, according to data from an autopsy study, classifies DOMV according to the location of the accessory orifice location into an anterolateral commissure, a posteromedial commissure, and without an accessory orifice (orifices of equal size) (1). A DOMV usually occurs congenitally; though, there is growing evidence that mitral annular calcification and MV intervention, particularly MV edge-to-edge repair and MV clip, can cause acquired DOMV (4-6). Half of the patients with DOMV have normal valvular function while the other half have a certain degree of valvular dysfunction including MR and MS (1). MR can be due to the abnormal orifice of MV itself or occur secondary to ruptured chordae tendineae as in our first case (7). Associated anomalies of DOMV can be classified into shunt lesions (e.g., atrioventricular canal defect—the most common association, atrial septal defect, ventricular septal defect, and patent ductus arteriosus), valvular lesions (e.g., double orifice tricuspid valve and Ebstein’s anomaly), and obstructive left heart lesions (e.g., bicuspid aortic valve and coarctation of the aorta) (1). To the best of our knowledge, there was no previous report regarding the pathophysiological relevance between DOMV and frequent RVOT premature ventricular beats as a presenting symptom like our second patient.
Given its availability and precision, the echocardiogram, including a 3-dimensional technique and transesophageal approach, is the mainstay of the investigation for a DOMV. The echocardiogram is used not only for diagnosis, classification, and function evaluation but also for associated anomalies assessment (8). The parasternal short-axis view is the best view for evaluating a DOMV; however, the parasternal long-axis and apical views are essential for assessing subvalvular apparatus, degree of regurgitation, and stenosis (9). CMR imaging, although not a mandatory investigation, can be helpful in case of an uncertain diagnosis and suspicion of associated anomalies (10,11).
The treatment strategies individually depend on the symptoms of each patient. Conservative management with regular clinical and imaging follow-up is the strategy of choice for the patient with no or minimal symptoms. The surgical intervention (e.g., MV repair and MV replacement) is indicated when the valvular dysfunction causes significant symptoms. In addition, surgical correction of associated anomalies is often required (1,12-14).
Conclusions
We described two patients with DOMV who presented with different disease severity resulting in different treatment decisions. These cases highlight the diversity of manifestations in patients with DOMV and the importance of appropriate investigations to evaluate valvular pathology and contemplating the treatment strategy.
Acknowledgments
The authors acknowledge the support for article processing from Cardiac Center, King Chulalongkorn Memorial Hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-52/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-52/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-52/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baño-Rodrigo A, Van Praagh S, Trowitzsch E, et al. Double-orifice mitral valve: a study of 27 postmortem cases with developmental, diagnostic and surgical considerations. Am J Cardiol 1988;61:152-60. [Crossref] [PubMed]
- Banerjee A, Kohl T, Silverman NH. Echocardiographic evaluation of congenital mitral valve anomalies in children. Am J Cardiol 1995;76:1284-91. [Crossref] [PubMed]
- Trowitzsch E, Bano-Rodrigo A, Burger BM, et al. Two-dimensional echocardiographic findings in double orifice mitral valve. J Am Coll Cardiol 1985;6:383-7. [Crossref] [PubMed]
- Bhamra-Ariza P, Muller DW. The MitraClip experience and future percutaneous mitral valve therapies. Heart Lung Circ 2014;23:1009-19. [Crossref] [PubMed]
- Oda T, Kono T, Akaiwa K, et al. Edge-to-edge repair for mitral regurgitation associated with isolated double-orifice mitral valve. Interact Cardiovasc Thorac Surg 2018;26:529-31. [Crossref] [PubMed]
- Lange M, Bültel H, Wichter T. Calcified mitral stenosis imitates a MitraClip(®) and forms a double orifice. Eur Heart J Case Rep 2018;2:yty084. [Crossref] [PubMed]
- Tomita Y, Yasui H, Tominaga R. Mitral valve repair for isolated double-orifice mitral valve with torn chordae. Ann Thorac Surg 1997;64:1831-4. [Crossref] [PubMed]
- Kutty S, Colen TM, Smallhorn JF. Three-dimensional echocardiography in the assessment of congenital mitral valve disease. J Am Soc Echocardiogr 2014;27:142-54. [Crossref] [PubMed]
- Silverman NH. Echocardiography of congenital mitral valve disorders: echocardiographic-morphological comparisons. Cardiol Young 2014;24:1030-48. [Crossref] [PubMed]
- Marcu CB, Beek AM, Ionescu CN, et al. Double orifice mitral valve visualized on echocardiography and MRI. Neth Heart J 2012;20:380-1. [Crossref] [PubMed]
- Laccetta G, Napoli AM. Incidental Finding of an Isolated Double-orifice Mitral Valve in an Asymptomatic Man. J Cardiovasc Echogr 2016;26:100-3. [Crossref] [PubMed]
- Congiu S, Josa M, Freixa X, et al. Mitral insufficiency with a double-orifice mitral valve in an adult patient. J Thorac Cardiovasc Surg 2007;134:250-1. [Crossref] [PubMed]
- Zhu D, Chen A, Zhao Q. Surgical repair for isolated congenital double-orifice mitral valve. Eur J Cardiothorac Surg 2011;39:268-70. [Crossref] [PubMed]
- Dogan OF, Demircin M, Karagoz T. Diagnosis and management of the double orifice mitral valve: three case reports. Heart Surg Forum 2005;8:E236-40. [Crossref] [PubMed]
Cite this article as: Theerasuwipakorn N, Chattranukulchai P, Satitthummanid S, Tumkosit M, Ongcharit P, Puwanant S, Boonyaratavej S. Double orifice mitral valve: a report of two cases with different severity and treatment. AME Case Rep 2023;7:12.


