
A woman with Sjogren’s syndrome and a new diagnosis of Evans syndrome: a case report
Highlight box
Key findings
• This case demonstrates that ES, a rare hematological disease, can be seen in Sjogren’s. It is imperative to consider this diagnosis in Sjogren’s patients with mild hematological symptoms and obtain an appropriate workup to evaluate for hemolysis and exclude other common etiologies of similar presentations.
What is known and what is new?
• ES is considered more challenging to treat due to decreased responsiveness to standard therapies, more frequent relapses, and higher mortality in comparison to isolated warm AIHA or ITP. Prompt identification of ES can lead to appropriate therapy selection and rapid hematological improvement as evidenced in this case.
What is the implication, and what should change now?
• Although the association of ES with Sjogren’s is uncommon, further research is necessary to understand the associated clinical characteristics, determine prognosis, and provide management recommendations.
Introduction
Evans syndrome (ES) is a rare disease characterized by the simultaneous or sequential development of autoimmune hemolytic anemia (AIHA) with immune thrombocytopenia (ITP), and less frequently autoimmune neutropenia. The association of ES with other diseases, including hematological malignancies, primary immunodeficiencies, and autoimmune diseases, can affect prognosis and management options, as cytopenias have been observed to be more severe in secondary versus primary disease (1,2). Of the autoimmune diseases associated with ES, systemic lupus erythematosus (SLE) was the most reported. Although recent studies describe the clinical characteristics of secondary ITP associated with primary Sjogren’s syndrome (SS) (3,4), the association with ES is rarely reported. We present a case of a 48-year-old woman with SS presenting with a new diagnosis of ES in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-3/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 48-year-old woman with a history of primary SS, uterine fibroids, and morbid obesity (body mass index 61.7 kg/m2) presented to the emergency department for urgent evaluation of acute thrombocytopenia (platelets 9×103/µL) noted on routine laboratory studies at her rheumatology visit. She was diagnosed with SS 2 years prior with a positive antinuclear antibody (ANA 1:640, speckled pattern), anti-SSA, anti-SSB, and sicca symptoms. Her complete blood count (CBC) had been within normal limits for 10 years prior, including when last checked 6 months prior to presentation. On admission, she noted one year of easy bruising, which became larger and more widespread with time, along with heavier menses in the setting of known uterine fibroids on Depo-Provera therapy. She denied other sources of bleeding or newly prescribed medications. The physical exam was notable for scattered ecchymoses over her bilateral upper and lower extremities.
Laboratory testing (Table 1) revealed normocytic anemia [hemoglobin (Hgb) 10.4 g/dL, mean corpuscular volume (MCV) 82.4 fL] and severe thrombocytopenia (platelets 9×103/µL). Laboratory studies were also significant for haptoglobin <5 mg/dL, lactate dehydrogenase (LDH) 257 U/L, and reticulocyte count 1.9%. Peripheral smear (Figure 1) demonstrated scattered spherocytes and decreased platelet count, without clumping. Direct antiglobulin testing (DAT) was positive and further eluate testing revealed warm autoantibodies. Notable negative studies included dsDNA antibody, smith antibody, rheumatoid factor, cyclic citrullinated peptide (CCP), human immunodeficiency virus (HIV), and hepatitis serologies, along with an unremarkable abdominal ultrasound.
Table 1
| Laboratory test | Initial presentation | 3-week follow-up | 6-week follow-up | Reference range |
|---|---|---|---|---|
| WBC (×103/µL) | 6.1 | 16.49 | 15.97 | 4.22–10.33 |
| RBC (m/µL) | 4.25 | 4.01 | 4.39 | 3.93–5.22 |
| Hemoglobin (g/dL) | 10.4 | 10.3 | 11.5 | 11.2–15.7 |
| Hematocrit (%) | 35 | 33.7 | 37.9 | 34.1–44.9 |
| MCV (fL) | 82.4 | 84 | 86.3 | 79.4–94.8 |
| MCH (pg) | 24.5 | 25.7 | 26.2 | 26.5–32.6 |
| MCHC (g/dL) | 29.7 | 30.6 | 30.3 | 32.3–36.5 |
| RDW-CV (%) | 15.1 | 17.4 | 15.9 | 11.4–14.4 |
| Platelets (×103/µL) | 9 | 282 | 305 | 160–383 |
WBC, white blood cell; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW-CV, red cell distribution width.
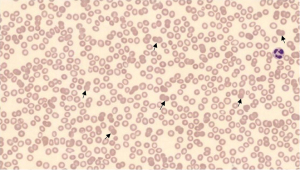
Given her clinical and laboratory findings, the patient was diagnosed with ITP and AIHA, which was confirmed by a very low haptoglobin level, mild spherocytes on peripheral smear and positive DAT. Eluate testing was also positive leading to a diagnosis of ES. A thorough workup, as included above, eliminated other common etiologies including thrombotic thrombocytopenic purpura (TTP), drug-induced thrombocytopenia, infectious etiologies (hepatitis C virus, HIV), liver disease, malignancy, and other autoimmune disease (i.e., SLE).
She received Dexamethasone 40 mg daily for 4 days concurrent with 2 days of IVIG 95 g with marked improvement in her thrombocytopenia (91×103/µL) by the end of the treatment course above. She was discharged on Prednisone 100 mg daily with arranged Hematology and Rheumatology follow-up. Her platelet count normalized within one month from her initial diagnosis (284×103/µL) followed by her Hgb level (11.5 g/dL) at 6 weeks post-diagnosis. A mild leukocytosis was noted at follow-up visits (Table 1) although attributed to the marginalization effect of glucocorticoids given the absence of infectious signs or symptoms. She was continued on a prednisone taper for 6 months. At follow-up visits, approximately one year after diagnosis, her cell counts remain within normal limits and the peripheral smear demonstrates normal platelet and red blood cell morphologies, without evidence of platelet clumping or schistocytes.
Discussion
ES, the simultaneous or sequential development of AIHA with ITP or autoimmune neutropenia, can be a rare manifestation of autoimmune disease. The pathophysiology of ES remains unknown although recent studies propose deficiencies of gene mutations, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), lipopolysaccharide responsive beige-like anchor protein (LRBA), tripeptidyl peptidase 2 (TPP2), or a decreased CD4/CD8 ratio which could be associated with the known immune dysregulation of the disease (5). The literature regarding ES in autoimmune diseases is limited. Furthermore, very little is known about ES in patients with Sjogren’s. In a case series of 68 patients with ES, approximately 50% of the cases were primary ES and the other 50% were secondary to underlying conditions such as lymphoproliferative disorders, immunodeficiency, or autoimmune disease (6). Of the individuals with autoimmune disease, seven patients had SLE and only two patients had SS. In another series of 116 adults with ES, none of the individuals had known SS (1).
Presentations of ES can vary, although cytopenias are typically more severe when associated with ES when compared with AIHA or ITP alone (1,2). Hematological abnormalities can be seen in SS and often may develop prior to the development of sicca symptoms or as the sole clinical manifestation of SS (7). In addition, the management of ES is challenging as recommendations are extrapolated from guidelines for isolated AIHA and ITP due to the disease’s rarity and many patients are refractory to the standard treatments (1,2). Corticosteroids and/or intravenous immunoglobulin (IVIG) are the most commonly used first-line therapies, with a typically longer steroid course duration of approximately 6 months when AIHA is present. IVIG is often added if the platelet count is <20,000/µL and is used more frequently in ES compared to patients with isolated AIHA (8). Rituximab or splenectomy may be considered in patients refractory to the standard treatment or if steroid-dependent (1,2). Even with treatment for secondary ES, responses can vary, relapses are common, and mortality is high with an approximate 5-year survival of 38% (9).
This case provides a rare example of a woman with SS presenting with mild bleeding symptoms and severe thrombocytopenia. In addition, a very low haptoglobin, spherocytes on peripheral smear, positive DAT, and positive eluate testing confirmed a warm AIHA, and the patient was diagnosed with ES in the setting of SS. Further testing ruled out alternative hematological, infectious, and autoimmune diagnoses. Following the initiation of IVIG and steroid therapy, her hematological function markedly improved, and she has remained in remission since. The strengths of this case report include rapid identification of ES, appropriate therapy selection, improvement in hematological function, and prompt recovery. In addition, the patient’s follow-up care within the medical system has allowed for long-term monitoring of the resolution of symptoms. For this reason, it is imperative to consider a diagnosis of ES in Sjogren’s patients presenting with hematological manifestations.
Conclusions
Very little is known about ES in patients with Sjogren’s and the literature regarding ES in AI diseases is limited. Although the association of ES with Sjogren’s is uncommon, cytopenias can be seen in SS and may often develop prior to sicca symptoms or as the sole clinical manifestation of the disease. Given the rarity of ES in SS, further research is necessary to understand the associated clinical characteristics, determine prognosis, and provide management recommendations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-3/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-3/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Audia S, Grienay N, Mounier M, et al. Evans' Syndrome: From Diagnosis to Treatment. J Clin Med 2020;9:3851. [Crossref] [PubMed]
- Shaikh H, Mewawalla P. Evans Syndrome. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing; 2022.
- Liu Y, Chen S, Sun Y, et al. Clinical characteristics of immune thrombocytopenia associated with autoimmune disease: A retrospective study. Medicine (Baltimore) 2016;95:e5565. [Crossref] [PubMed]
- Dai F, Yang G, Rao P, et al. Clinical Characteristics of Secondary Immune Thrombocytopenia Associated With Primary Sjögren's Syndrome. Front Med (Lausanne) 2020;7:138. [Crossref] [PubMed]
- Jaime-Pérez JC, Aguilar-Calderón PE, Salazar-Cavazos L, et al. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med 2018;9:171-84. [Crossref] [PubMed]
- Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood 2009;114:3167-72. [Crossref] [PubMed]
- Meena DS, Bohra GK. Primary Sjogren's syndrome presenting as autoimmune cytopenia. Clin Pract 2019;9:1190. [Crossref] [PubMed]
- Flores G, Cunningham-Rundles C, Newland AC, et al. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia: results in 73 patients. Am J Hematol 1993;44:237-42. [Crossref] [PubMed]
- Hansen DL, Möller S, Andersen K, et al. Evans syndrome in adults - incidence, prevalence, and survival in a nationwide cohort. Am J Hematol 2019;94:1081-90. [Crossref] [PubMed]
Cite this article as: Ghebranious MA, Eseddi J, Galous H. A woman with Sjogren’s syndrome and a new diagnosis of Evans syndrome: a case report. AME Case Rep 2023;7:25.


