
Two primary cancers appeared after discontinuation of nivolumab in the course of treating Hodgkin lymphoma: a case report
Highlight box
Key findings?
• Nivolumab, an immune checkpoint inhibitor (ICI) used for relapse refractory Hodgkin’s lymphoma in our patient was simultaneously suppressing two other primary malignancies of Merkle cell carcinoma and squamous cell carcinoma of the tongue. Discontinuation of nivolumab exposed these malignancies in a short period of time.
What is known and what is new?
• ICIs like nivolumab have demonstrated anti-tumor efficacy in several cancers to date by blocking the programmed death-1 (PD-1) pathway and promoting antitumor immune responses.
• Their efficacy on different concurrent malignancies has not been studied till date, due to paucity of such cases. Our case report showcases the broad activity of nivolumab against two malignancies in addition to primary Hodgkin’s lymphoma, discontinuation of which revealed them.
What is the implication and what should change now?
• Our report brings attention to the possibility of uncovering new malignancies after discontinuation of ICIs in high-risk patients and reinforces the importance of close follow up in patients at high risk of developing multiple malignancies.
Introduction
The discovery of immune checkpoint proteins such as programmed death-1 (PD-1)/PD-L1 and CTLA-4 has revolutionized the field of cancer immunotherapy (1). Immune checkpoint inhibitors (ICIs) against these proteins, block signaling through the PD-1 pathway, release inhibition of T cells, and augment antitumor immune responses. Nivolumab is one such humanized IgG4 monoclonal anti-PD-1 antibody approved by the US Food and Drug Administration (FDA) in metastatic melanoma, renal cell carcinoma, gastric or gastroesophageal junction cancer, hepatocellular, classical Hodgkin’s lymphoma, head and neck and non-small cell lung cancers (1,2).
Although there are many ongoing clinical trials studying the effect of nivolumab in individual cancers, its efficacy on different concurrent malignancies in the body has not been studied till date, due to paucity of such cases. Here we report a unique case of a patient with relapse refractory Hodgkin’s lymphoma when discontinued on long term maintenance with nivolumab due to relapse of the disease, then exhibited two different primary malignancies of Merkle cell carcinoma and squamous cell carcinoma (SCC) of the tongue in a short duration. Although nivolumab has been demonstrated to have anti-tumor activity in head and neck cancers, there has been only one clinical case report to date (3), that reported the antitumor activity of nivolumab on Merkle cell carcinoma. We intend to add to the literature this notable finding of tumor suppressive effect of nivolumab on three different malignancies simultaneously. We present the following case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-87/rc).
Case presentation
Our patient is a 75-year-old Caucasian male with past medical history of insulin dependent diabetes mellitus, hypertension, and family history significant for lung cancer in mother who passed at age 48. He was first diagnosed of Hodgkin’s lymphoma at the age of 58 in October 2005, when he presented with fatigue, night sweats, hives, and weight loss of 25 pounds. Physical examination revealed cervical and axillary lymphadenopathy. Complete blood count (CBC) showed hemoglobin of 10.1 g/dL, white blood cell (WBC) count of 15,000/µL (range, 4,000–11,000/µL) and platelet count of 561,000/µL (range, 150,000–450,000/µL), erythrocyte sedimentation ratio (ESR) of 140 mm/h (range, 0–22 mm/h), and lactate dehydrogenase (LDH) of 353 U/L (range, 140–280 U/L). Biopsy of an enlarged right cervical lymph node revealed classic HL, nodular sclerosis subtype. Bone marrow biopsy showed cellular bone marrow with trilinear hematopoiesis without evidence of HL. Disease was initially staged as IVb due to extra nodal involvement.
The patient was initiated on ABVD regimen (adriamycin-bleomycin-vinblastine-dacarbazine), which he received for 7 cycles as he refused the 8th cycle. Positron emission tomography-computed tomography (PET-CT) in March 2006 post completion of ABVD regimen (doxorubicin at the dose of 25 mg/m2, bleomycin at 10 mg/m2, vinblastine at 6 mg/m2, and dacarbazine at 375 mg/m2) showed complete response (Figure 1). Eight months post treatment, surveillance PET-CT in November 2006 showed relapse of disease. Salvage chemotherapy with RICE [rituximab at 375 mg/m2, ifosfamide at 5,000 mg/m2, carboplatin area under the curve (AUC) of 5, etoposide at 100 mg/m2] was given for two cycles as the disease was CD20+. The patient underwent preparation for autologous stem cell transplant with BEAM (carmustine, etoposide, cytarabine, melphalan) per standard protocol and subsequently was transplanted in April 2007 with complete response. PET-CT in December 2007 showed no evidence of relapsed disease and patient has been in remission until July 2011. PET-CT scan at this time showed enlarged lymphadenopathy above and below diaphragm suggestive of recurrence. The patient was informed of the poor prognosis at this point given the relapse/refractory nature of his disease and was initiated on gemcitabine at 1,000 mg/m2 and navelbine at 30 mg/m2 in November 2011. After completion of 8 cycles, PET-CT to assess response showed residual disease. Upon discussion of options, patient refused allogenic stem cell transplant and expressed interest to take a break from chemotherapy.
On surveillance, progression of disease was noted 7 months later in November 2012. Brentuximab dosed at 1.8 mg/kg every 3 weeks, was started as next line of salvage chemotherapy in February 2013, which was eventually discontinued due to intolerable grade II neuropathy despite medications. PET-CT post treatment showed partial response (PR) in May 2013 and was continued on surveillance. Subsequently, patient was initiated on everolimus (10 mg p.o. daily) upon evidence of progression of disease in October 2013. Patient had multiple episodes of C. difficile infection due to which everolimus was discontinued intermittently. Even though it was restarted later, disease progression was noted in October 2014.
As part of a clinical trial, the patient was later started on nivolumab 240 mg intravenous (IV) every 2 weeks in November 2014, and the disease was in PR. Six years later in June 2020, surveillance PET-CT showed hypermetabolic activity in the right lateral border of the tongue, biopsy of which identified SCC. He underwent right partial glossectomy and lymph node dissection which showed a 3.1 cm SCC of right tongue with clear margins and no lymph nodal involvement. It was determined that patient does not require any further adjuvant chemoradiation. His course has been eventless since then with no relapse of HL on surveillance imaging, until 2 years later when he was hospitalized for presyncope in January 2022. CT thorax/abdomen/pelvis showed progressed disease and a large pleural effusion, cell cytology from thoracocentesis showed relapse of HL. Nivolumab was discontinued due to progression of disease and the patient was again started on bendamustine at 60 mg/m2 in January 2022.
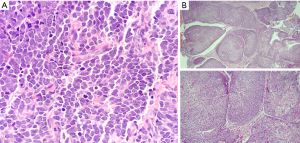
A month later in February 2022, the patient visited his dermatologist as he noted an enlarging right ear lesion. A suspicious dome-shaped mass in the scaphoid fossa of the right ear measuring 9×8×5 mm was noticed and biopsied. Pathology revealed Merkel cell carcinoma (MCC) (Figure 2A). Patient underwent wide local excision and sentinel lymph node biopsy in April 2022. Triple scope performed during the procedure with laryngoscopy, bronchoscopy and esophagogastroduodenoscopy revealed an incidental 5×5 mm exophytic mass on the right base of tongue, biopsy of which was found to be invasive SCC (Figure 2B). A prior PET-CT scan from a month ago, identified a non-enlarged left cervical level 2A lymph node with standardized uptake value (SUV) of 2.4, which was initially thought to be reactive in nature. Histopathology of the lymph node biopsy confirmed metastatic SCC, p16−. The patient was staged as T1N2M0. Due to two concomitant cancers, the patient was started on concurrent chemoradiation therapy to both areas.
Over the course of years, patient was diagnosed of several other medical conditions such as coronary artery disease with multiple stent placements, peripheral arterial disease requiring femoral-tibial trunk bypass, residual neuropathy, and recurrent complicated urinary tract infections renal due to stones. Due to these comorbidities and older age, patient was started on deescalated dose of carboplatin to an AUC of 2. Bendamustine for relapsed refractory HL was held, while the patient is currently completing chemoradiation. It is to be noted that withdrawal of nivolumab almost immediately resulted in increase of the size of his unknown MCC of the right ear lobe. It also likely led to relapse of his prior localized SCC of the tongue with metastasis.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
Classical Hodgkin Lymphoma (cHL) is a malignancy of the lymphatic system with an incidence of 2–3 cases per 100,000 people every year in developed countries (3). Since 1990, ABVD has become the standard chemotherapy regimen for patients with classical HL because of its efficacy and lower toxicity than other agents (4). Immunotherapies are other modalities of treatment.
ICIs are a class of drugs that block proteins that upregulate immune responses. Currently, immunotherapy is most used in recurrent or relapsed cases of HL, many studies have also proven it to be beneficial as a first line treatment (5-7). PD-1 inhibitors are currently clinically approved for use in numerous solid tumor malignancies. These agents bind to the PD-1 receptor or the ligand and prevent the death of the T-lymphocytes to prevent the development of immunologic tolerance to the tumor and therefore potentiate immune destruction of the tumor (8).
Nivolumab is a human monoclonal antibody with high affinity to PD-1 and blocks interactions of PD-1 with both PD-L1 and PD-L2 and stimulates tumor antigen-specific T cell to develop appropriate immune response against cancer cells for its destruction. It was first approved by FDA in the treatment of melanoma and gradually multiple trials have proven its efficacy as first line or in later lines of treatment in various other tumors (9). Nivolumab was also recently approved by FDA to treat relapsed/refractory cHL after autologous hematopoietic cell transplantation (Auto-HCT) (10). Nivolumab use has been studied in metastatic non-small cell and small cell lung cancer, advanced renal cell cancer, recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN), microsatellite instability-high or mismatch repair deficient solid tumors or colorectal cancer (11).
Pembrolizumab (PD-1 inhibitor) and nivolumab are the first ICIs approved for the treatment of recurrent/metastatic SCCHN, FDA approval was in August and November 2016 respectively. Based on the results of Checkmate 141 clinical trial, nivolumab use when compared to chemotherapy of investigator’s choice resulted in a longer overall survival (OS) in this category of patients (12). In 2018, 2-year long-term survival update of the trial redemonstrated OS benefit with nivolumab regardless to PD-L1 expression or human papillomavirus (HPV) status (13). Also, in 2022, a long-term subgroup analysis of the trial was published and reported improved OS for patients who received nivolumab use as first line treatment for metastatic SCCHN (14). To date, studies are lacking on the role of ICI including nivolumab as a first line postoperatively in early stages SCCHN. After his SCC tongue resection, our patient was already on nivolumab maintenance for his HL, which could have prevented tongue cancer’s progression. This can open the door for future trials to assess ICI roles in early stages of SCCHN.
Because MCC is an immunologically sensitive cancer, the availability of ICI has altered the therapeutic options for advanced MCC (14). The first ICI to demonstrate objective tumor regression was pembrolizumab, which was added to the National Comprehensive Cancer Network (NCCN) recommendations for the treatment of disseminated MCC because of a phase II trial’s findings (15). The first FDA-approved medication for MCC, avelumab (a PD-L1 inhibitor), was introduced in March 2017 and has significantly better outcomes than chemotherapy (16,17). Despite the limited number of patients needed to attain statistical significance, the Checkmate 358 study, which evaluated the safety and effectiveness of nivolumab and nivolumab combination in virus-associated malignancies including MCC, revealed a 64% overall response rate (ORR) for MCC (18).
In our case report, we describe the unique observation of nivolumab suppressing the growth of two separate malignancies apart from the primary malignancy, discontinuation of which has then contributed to their growth and subsequent diagnosis. Literature review does not show any similar reported cases so far. It is to be noted that, due to multiple prior lines of chemotherapy for his relapse refractory Hodgkin’s lymphoma and as part of autologous stem cell transplant, our patient is at very high risk of development of secondary cancers. The history of SCC tongue also indicates a baseline immune dysfunction which could predispose him to secondary cancers. This case cautions physicians to keep in view the possibility of developing new cancers in patients who are at high risk. Close follow up with regular routine physical examination is highly recommended in patients at high-risk for malignancies, including but not limited to significant family history for multiple malignancies, prior exposure to toxins or chemotherapy among others. Nivolumab, a systemic immunomodulator has been suppressing the other malignancies in addition to HL from progression, in our patient who seems to be inherently at risk of multiple malignancies due to unknown reasons.
Our case report is limited to presentation of a single patient, as it very uncommon to have multiple malignancies in a patient. Although there are many ongoing clinical trials studying the effect of nivolumab in individual cancers, its efficacy on different concurrent malignancies in the body has not been studied till date, due to paucity of such cases. We do think it is worth reporting as it can add to the current literature on the possible association between ICI use and masking possible undiagnosed tumors.
Conclusions
Nivolumab could mask other primary tumors while being used for the treatment of an entirely different type of cancer, as in our patient, who was diagnosed with Merkle cell carcinoma and metastatic tongue SCC after cessation of nivolumab for relapse/refractory HL. Identification and reporting of such cancers is uncommon, as they could be easily masked by prolonged use of immunotherapies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-87/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-87/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol 2022;29:3044-60. [Crossref] [PubMed]
- Ramchandren R, Domingo-Domènech E, Rueda A, et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J Clin Oncol 2019;37:1997-2007. [Crossref] [PubMed]
- Walocko FM, Scheier BY, Harms PW, et al. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer 2016;4:79. [Crossref] [PubMed]
- Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol 2000;13:193-207. [Crossref] [PubMed]
- Armand P, Engert A, Younes A, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol 2018;36:1428-39. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol 2003;21:607-14. [Crossref] [PubMed]
- Renner C, Stenner F. Cancer Immunotherapy and the Immune Response in Hodgkin Lymphoma. Front Oncol 2018;8:193. [Crossref] [PubMed]
- Guo L, Zhang H, Chen B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J Cancer 2017;8:410-6. [Crossref] [PubMed]
- Donato EM, Fernández-Zarzoso M, De La Rubia J. Immunotherapy for the treatment of Hodgkin lymphoma. Expert Rev Hematol 2017;10:417-23. [Crossref] [PubMed]
- LaFleur MW, Muroyama Y, Drake CG, et al. Inhibitors of the PD-1 Pathway in Tumor Therapy. J Immunol 2018;200:375-83. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45-51. [Crossref] [PubMed]
- Gillison ML, Blumenschein G, Fayette J, et al. Long-term Outcomes with Nivolumab as First-line Treatment in Recurrent or Metastatic Head and Neck Cancer: Subgroup Analysis of CheckMate 141. Oncologist 2022;27:e194-8. [Crossref] [PubMed]
- Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 2016;374:2542-52. [Crossref] [PubMed]
- Chin K, Chand VK, Nuyten DSA. Avelumab: clinical trial innovation and collaboration to advance anti-PD-L1 immunotherapy. Ann Oncol 2017;28:1658-66. [Crossref] [PubMed]
- Becker J, Lorenz E, Haas G, et al. Evaluation of real world treatment outcomes in patients with metastatic merkel cell carcinoma (MCC) following second line chemotherapy. Ann Oncol 2016;27:abstr VI397.
- Topalian SL, Bhatia S, Hollebecque A, et al. Abstract CT074: non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC). Cancer Res 2017;77:CT074.
Cite this article as: Potluri LB, Nanjareddy S, Al Sbihi A, Manasrah N, Smith W 4th, Sano D. Two primary cancers appeared after discontinuation of nivolumab in the course of treating Hodgkin lymphoma: a case report. AME Case Rep 2023;7:30.


