
Chlamydia psittaci causing severe pneumonia with an initial complaint of massive watery sputum: a case report
Highlight box
Key findings
• It needs intensive treatment in severe C. psittaci pneumonia with initial complaint of massive watery sputum.
What is known and what is new?
• The symptom of non-productive cough in C. psittaci pneumonia has been often reported.
• In severe C. psittaci pneumonia with massive watery sputum, which results from massive inflammation, the treatment of effective endotracheal suction and corticosteroids, is required in addition to the targeted antimicrobial therapy.
What is the implication, and what should change now?
• Providing timely and comprehensive treatment is important for severe C. psittaci pneumonia and acute respiratory distress syndrome patients admitted to the intensive care unit and could improve outcomes.
Introduction
Psittacosis, also known as avian chlamydiosis, ornithosis, and parrot fever, is caused by the zoonotic bacterium Chlamydia psittaci (C. psittaci), which can be transmitted to humans primarily from birds (1). Psittacosis is a systemic disease, and the spectrum of the disease is highly variable, ranging from subclinical infection to less commonly reported severe pneumonia requiring mechanical ventilation or multi-organ failure. C. psittaci pneumonia comprises approximately 1% of all community acquired pneumonia (CAP) cases (2). However, based on metagenomic next-generation sequencing (mNGS), it accounts for 8% of severe CAP (SCAP) cases, who are immunocompetent and admitted to the intensive care unit (ICU) (3), and can be life-threatening in severe cases due to failure to diagnose or identify the pathogen in time and delayed treatment with targeted antibiotics. Therefore, it is necessary to highlight the optimal management of severe C. psittaci pneumonia to improve patient outcomes.
Here, we report a case of severe C. psittaci pneumonia with massive watery sputum, which is not specially reported before, and the timely pathogen detection through mNGS and comprehensive treatments are important for dealing with severe C. psittaci pneumonia patients admitted to ICU. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-88/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the research ethics board of Sun Yat-sen Memorial Hospital (Guangzhou, China) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 51-year-old man was admitted to the emergency department because of recurrent productive cough for 1-month, accompanied by fever and dyspnea for 4-hour. He initially coughed watery sputum, which subsequently became viscous and yellow. His temperature was 40 ℃, heart rate was 110/min (relative bradycardia), blood pressure was 110/70 mmHg, respiratory rate was 32/min, and oxygen saturation was 95% with 8 L oxygen supplementation. He appeared somnolent with limb weakness, but did not have chills, muscle soreness, or nausea. He developed progressive dyspnea (respiratory rate 40/min) with weak expectoration, and the oxygen saturation dropped to 70%. Hence, the patient immediately received tracheal intubation and mechanical ventilation, and was treated with febrifuge, imipenem/cilastatin sodium, and levofloxacin. After undergoing a chest computed tomography (CT) scan, he was immediately transferred to the ICU for further treatment.
The patient had a history of hyperthyroidism. His thyroid function had returned to normal after treatment (details unknown) and he had stopped taking drugs for 2 years, without a repeat thyroid function test. He was non-smoker and did not drink alcohol, and had no history of tuberculosis (TB), lung cancer, diabetes mellitus, hypertension, coronary heart disease, trauma, or psychiatric disease. The patient was a resident of Guangdong province, southern China. He had been in contact with pigeons around his living environment 1 year ago, but had not been in direct contact with any birds, poultry, or horses over the past year.
When he was admitted to ICU (day 1, D1), his vital signs were normal, and suppressed breath sounds in the left lower lung and wet rales in bilateral lower lungs were heard on auscultation. Laboratory data upon admission to the ICU revealed white blood cell (WBC) count 7.36×109/L, with an elevated neutrophil ratio of 93.3%. The plasma concentrations of C-reactive protein (CRP) and procalcitonin (PCT) were 311.57 mg/L and 3.02 ng/mL, respectively. Serum biochemical tests revealed that aspartate aminotransferase (AST) was 178 U/L, alanine aminotransferase (ALT) was 74 U/L, total bilirubin (TBil) was 29.5 µmol/L, and creatine phosphokinase (CK) was 6,962 U/L. Arterial blood gas analysis showed a pH of 7.428, arterial partial pressure of oxygen (PaO2) of 112.6 mmHg, arterial partial pressure of carbon dioxide (PaCO2) of 22.2 mmHg, oxygenation index of 281.5 mmHg, and lactate of 1.1 mmol/L. Yellow viscous sputum was aspirated from the left lower lung via bronchofibroscopy, and treatment with empirical anti-bacterial agents piperacillin-tazobactam and azithromycin was initiated.
Despite this treatment, the patient’s condition deteriorated rapidly, with recurrent high fever of 40.3 ℃ and relative bradycardia on D3. Moreover, he suffered respiratory distress (respiratory rate 50/min) and a large volume of yellow watery sputum was aspirated by the endotracheal tube (Figure 1A) from right lobes and left upper lobe, confirmed by bronchofibroscopy. The electrolytes in the watery sputum were similar to plasma electrolytes (Table 1), indicating a leakage of plasma. The oxygenation index dropped to 52 mmHg, and the repeat chest X-ray showed progressive multiple patchy shadows on bilateral lungs, especially the right lung (Figure S1). He was suffering progressively severe hypoxemia with bilateral opacities in chest X-ray that excluding cardiac failure or fluid overload, acute respiratory distress syndrome (ARDS) was diagnosed according to Berlin definition (4). The serum biochemical tests showed an acute increase in creatinine. After integrative treatment with effective and timely intratracheal suction to maintain patency of air way and improve gas exchange, positive end-expiratory pressure (PEEP) increased from 8 to 15 cmH2O, and intravenous methylprednisolone (80 mg/day), vancomycin, sedation and short-term neuromuscular blocking agents (NMBAs) (12 hours) were used. The dyspnea was relieved, and the sputum became viscous after 2 days of treatment, along with a reduction in the volume of sputum on D5 (Figure 1B).
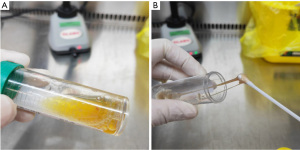
Table 1
| Composition | Plasma | Watery sputum |
|---|---|---|
| Na+ (mmol/L) | 141.6 | 146.2 |
| Cl− (mmol/L) | 114.8 | 119.7 |
| K+ (mmol/L) | 4.0 | 5.4 |
| Ca2+ (mmol/L) | 1.11 | 2.21 |
| TBil (µmol/L) | 30.7 | 25.7 |
TBil, total bilirubin.
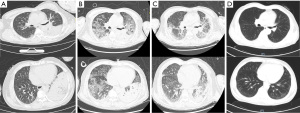
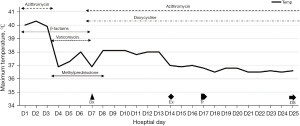
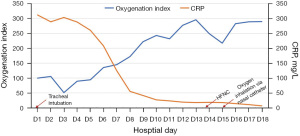
While the CT scan on D1 revealed a large air-space consolidation of left lower lung (Figure 2A), the chest CT on D9 showed the new onset ground-glass opacities and inflammatory exudates of the right lung and left upper lobe (Figure 2B). Unbiased mNGS of the bronchoalveolar lavage fluid (BALF) on D3 identified 165 sequence reads corresponding to C. psittaci, and there was no sequence read corresponding to other pathogens. Treatment with targeted anti-bacterial agents doxycycline (0.1 g bid orally) and azithromycin (0.5 g qd intravenous drop infusion) was initiated. After 8 days of targeted anti-bacterial therapy, a repeat CT scan on D15 showed absorption of the bilateral diffused patchy shadows, as well as consolidation of left lower lobe, along with minor pleural effusion (Figure 2C). The patient was extubated on D14, and the productive cough was alleviated, after which he was transferred to the respiratory department on D17 and discharged on D25 with oral medications (azithromycin and doxycycline for 2 weeks). He was followed up without complaint on D68 after ICU admission, when the repeat chest CT showed that the bilateral exudation and left lower lobe consolidation had almost disappeared, without pleural effusion (Figure 2D). A treatment summary is presented in Figure 3. The patient’s fever completely subsided on D14 (Figure 3). With the improvement of dyspnea and oxygenation index in arterial blood gas analysis (Figure 4), the CRP level (Figure 4) and the percent of neutrophil-granulocyte (Figure 5A) dropped steadily, and the biochemical indicators (TBil, Cr) returned to normal levels (Figure 5B), with CK of 56 U/L.

Besides the successful treatment of severe C. psittaci pneumonia and ARDS, the patient also had a history of hyperthyroidism. However, his thyroid function revealed hypothyroidism, and treatment with oral levothyroxine (12.5 mg/d) was initiated.
Discussion
Cunha (5) stated that differentiating atypical from typical CAP pathogens lies in the presence or absence of extra-pulmonary findings. Typical CAPs caused by Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis are characterized by clinical and laboratory findings limited to the lungs. In our cases, the patient presented with severe pneumonia and concurrent extra-pulmonary injuries. Hence, we initiated short-term treatment (3 days) with azithromycin to cover atypical pathogens, and subsequently added doxycycline (plus azithromycin) upon diagnosis of psittacosis on D7. Fulminant psittacosis can lead to multi-organ dysfunction, as observed in our patient who experienced acute hepatic and kidney injury. Fortunately, targeted antibiotics treatment resulted in their recovery within 1 week. Additionally, Su et al. (6) demonstrated that patients with severe C. psittaci pneumonia exhibited abnormal CK and brain natriuretic peptide (BNP) levels, and were more likely to develop dyspnea and progress to respiratory failure with involvement of multiple lung lobes. This finding aligns with our case.
Psittacosis can be diagnosed by real-time polymerase chain reaction (RT-PCR) or serology based on clinical suspicions. Recent studies (7-11) have reported the successful use of mNGS for diagnosing psittacosis. The mNGS of BALF (3) has the potential to improve pathogen identification in cases of severe pneumonia. The advantages of mNGS in detecting lung disease pathogens include its unbiased comprehensive profiling of microbial genomes, high throughput sequence, rapid results, and high sensitivity, even in patients previously treated with antibiotics. Additionally, BALF meets the requirements for accurate diagnosis of lung infections (12) and is feasible and accurate for patients undergoing mechanical ventilation. However, there are limitations to using mNGS, including (I) limited access to NGS platform due to technical requirement and high cost; (II) the challenge of obtaining qualified samples; (III) the potential detection of both pathogens and the airway microbiome; (IV) the need for a specific method for fungi DNA extraction when fungal infection is suspected based on clinical evidence. In our patient, mNGS successfully identified an atypical pathogen that was not detected in sputum/BALF cultures.
It is stated that co-infections are extremely rare, and it is unnecessary to use diagnostic resources to search for co-pathogens in patients with CAP (5). However, in our patient, it was important to distinguish between C. psittaci pneumonia and nosocomial infection due to the rapid progression of inflammation in other lobes after the left lower lobe. Despite the absence of other pathogens (including bacteria and fungi) in BALF or sputum cultures, the rapid new onset of exudation in the right lobe and left upper lobe (2 days after ICU admission), as well as the improvement of left lower lobe consolidation after targeted antimicrobial therapy against C. psittaci, clinically supported the diagnosis of C. psittaci infection. We believe that the worsening condition of the patient was a result of the infection spreading from left lower lobe to the right lung and left upper lobe.
While hyper-concentrated, dehydrated environments are typically associated with muco-obstructive diseases (13-15), some cases have reported excessive watery sputum in conditions such as bronchoalveolar carcinoma (BAC) or adenocarcinoma (16-19), infectious diseases (including TB, Aspergillosis) (20-22), poisoning (23,24), and bronchobiliary fistula (25). In the case of C. psittaci pneumonia, non-productive cough has often been reported (26-29). However, the patient in this report had been expectorating for 1 month, with initially watery sputum that subsequently became yellow and thick, this patten was similar to the characteristics of sputum in the ICU where a large volume of golden-yellow watery secretion exudated from the right and left upper lobar/segmental bronchi before becoming viscous. Hence, the initial watery sputum may be a characteristic of C. psittaci pneumonia that has not been previously reported.
The cause of watery sputum in C. psittaci pneumonia is not clear. Studies (30,31) have shown that the hydration of airway mucus is controlled by the active ion transport and water permeability of airway epithelia. We found that the ionic composition of watery sputum was similar to plasma, suggesting a leakage from plasma. Genotyping of C. psittaci is important for understanding its impact in humans (1). There are 10 genotypes (designated A to G, WC, E/B, and M56) of C. psittaci (32-37), with some found in humans (29,34,37,38). Our hypothesis is that severe epithelial inflammatory damage caused by C. psittaci may lead to watery sputum. Different genotypes of C. psittaci may cause different clinical symptoms. The production of a massive volume of watery sputum is uncommon in C. psittaci pneumonia, but can be serious to cause airway obstruction and severe respiratory distress. When the watery sputum become thick, it can cause lung consolidation, which may explain the consolidation of left lower lobe on the first chest CT on D1 in our patient. Treatment options to reduce the volume of water in the sputum include corticosteroids, macrolide antibiotics, and other medications (18,39). Given C. psittaci causes a more severe inflammatory reaction than other chlamydia species (35), corticosteroids may help reduce lung inflammation caused by C. psittaci. Previous studies (40-42) have shown the benefits of corticosteroids in severe C. psittaci-induced ARDS or organizing pneumonia. A recent randomized controlled trial (43) also indicates that hydrocortisone improves mortality in SCAP cases in ICU. In this report, we combined methylprednisolone with antibiotics to treat the hyper-inflammatory response caused by C. psittaci infection and improve the patient’s outcome.
Conclusions
In summary, in severe C. psittaci pneumonia, which often affects multiple lung lobes and organs, the prompt identification of the atypical pathogen through BALF mNGS may facilitate early use of effective antibiotics. The presentation of our patient underscores that the significance of timely and comprehensive treatment for severe cases of C. psittaci pneumonia and ARDS in ICU, as it can improve survival rates.
Acknowledgments
The authors thank the patient and his daughter, and the Da Zhou Center Hospital (Sichuan, China) for the repeated CT scan during the period of follow-up.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-88/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-88/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-88/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the research ethics board of Sun Yat-sen Memorial Hospital (Guangzhou, China) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Balsamo G, Maxted AM, Midla JW, et al. Compendium of Measures to Control Chlamydia psittaci Infection Among Humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. J Avian Med Surg 2017;31:262-82. [Crossref] [PubMed]
- Hogerwerf L, DE, Gier B, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect 2017;145:3096-105. [Crossref] [PubMed]
- Wu X, Li Y, Zhang M, et al. Etiology of Severe Community-Acquired Pneumonia in Adults Based on Metagenomic Next-Generation Sequencing: A Prospective Multicenter Study. Infect Dis Ther 2020;9:1003-15. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect 2006;12:12-24. [Crossref] [PubMed]
- Su S, Su X, Zhou L, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med 2021;10:8051-60. [Crossref] [PubMed]
- Yuan Y, Zhang X, Gui C. Detection of Chlamydia psittaci in both blood and bronchoalveolar lavage fluid using metagenomic next-generation sequencing: A case report. Medicine (Baltimore) 2021;100:e26514. [Crossref] [PubMed]
- Yang F, Li J, Qi B, et al. Clinical Symptoms and Outcomes of Severe Pneumonia Caused by Chlamydia psittaci in Southwest China. Front Cell Infect Microbiol 2021;11:727594. [Crossref] [PubMed]
- Xiao Q, Shen W, Zou Y, et al. Sixteen cases of severe pneumonia caused by Chlamydia psittaci in South China investigated via metagenomic next-generation sequencing. J Med Microbiol 2021; [Crossref] [PubMed]
- Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection 2020;48:535-42. [Crossref] [PubMed]
- Zhang H, Zhan D, Chen D, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med 2020;8:401. [Crossref] [PubMed]
- Liu BB, Tian Q, Wang P, et al. Evaluating the diagnostic value of using metagenomic next-generation sequencing on bronchoalveolar lavage fluid and tissue in infectious pathogens located in the peripheral lung field. Ann Palliat Med 2022;11:1725-35. [Crossref] [PubMed]
- Hill DB, Button B, Rubinstein M, et al. Physiology and pathophysiology of human airway mucus. Physiol Rev 2022;102:1757-836. [Crossref] [PubMed]
- Atanasova KR, Reznikov LR. Strategies for measuring airway mucus and mucins. Respir Res 2019;20:261. [Crossref] [PubMed]
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010;363:2233-47. [Crossref] [PubMed]
- Arriero JM, Chiner E, Signes-Costa J, et al. Chronic alveolar consolidation and watery sputum in an elderly woman. Respiration 2000;67:693-5. [Crossref] [PubMed]
- Hidaka N, Nagao K. Bronchioloalveolar carcinoma accompanied by severe bronchorrhea. Chest 1996;110:281-2. [Crossref] [PubMed]
- Rémi C, Rémi J, Bausewein C. Pharmacological Management of Bronchorrhea in Malignant Disease: A Systematic Literature Review. J Pain Symptom Manage 2016;51:916-25. [Crossref] [PubMed]
- Roeder NL, Marshall JD, Britto CJ. A Woman in Her 60s With Lung Adenocarcinoma Presents With Copious Watery Sputum and Respiratory Failure. Chest 2017;152:e143-6. [Crossref] [PubMed]
- Romero-Palacios A, Mera Gallardo O, Jiménez Aguilar P, et al. Rev Iberoam Micol 2019;36:34-36. [Obstructive tracheobronchitis due to Aspergillus fumigatus in an immunocompetent patient]. [Crossref] [PubMed]
- Kurahara Y. Massive Bronchorrhea. Intern Med 2021;60:2155-6. [Crossref] [PubMed]
- Olum R, Osaigbovo II, Baluku JB, et al. Mapping of Chronic Pulmonary Aspergillosis in Africa. J Fungi (Basel) 2021;7:790. [Crossref] [PubMed]
- Slavica V, Dubravko B, Milan J. Acute organophosphate poisoning: 17 years of experience of the National Poison Control Center in Serbia. Toxicology 2018;409:73-9. [Crossref] [PubMed]
- Weir AGA, Makin S, Breeze J. Nerve agents: emergency preparedness. BMJ Mil Health 2020;166:42-6. [Crossref] [PubMed]
- Cheng AC, Chen HW, Chen PJ, et al. Bronchobiliary Fistula. Intern Emerg Med 2021;16:1093-4. [Crossref] [PubMed]
- Shi Y, Chen J, Shi X, et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis 2021;21:621. [Crossref] [PubMed]
-
Ojeda Rodriguez JA Modi P Brady MF 2023 . - Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2000. Centers for Disease Control and Prevention. MMWR Recomm Rep 2000;49:3-17. [PubMed]
- Vande Weygaerde Y, Versteele C, Thijs E, et al. An unusual presentation of a case of human psittacosis. Respir Med Case Rep 2018;23:138-42. [Crossref] [PubMed]
- Jayaraman S, Song Y, Vetrivel L, et al. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest 2001;107:317-24. [Crossref] [PubMed]
- Schmidt H, Michel C, Braubach P, et al. Water Permeability Adjusts Resorption in Lung Epithelia to Increased Apical Surface Liquid Volumes. Am J Respir Cell Mol Biol 2017;56:372-82. [Crossref] [PubMed]
- Radomski N, Einenkel R, Müller A, et al. Chlamydia-host cell interaction not only from a bird's eye view: some lessons from Chlamydia psittaci. FEBS Lett 2016;590:3920-40. [Crossref] [PubMed]
- Geens T, Desplanques A, Van Loock M, et al. Sequencing of the Chlamydophila psittaci ompA gene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method. J Clin Microbiol 2005;43:2456-61. [Crossref] [PubMed]
- Van Lent S, Piet JR, Beeckman D, et al. Full genome sequences of all nine Chlamydia psittaci genotype reference strains. J Bacteriol 2012;194:6930-1. [Crossref] [PubMed]
- Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis 2015;73:1-15. [Crossref] [PubMed]
- Read TD, Joseph SJ, Didelot X, et al. Comparative analysis of Chlamydia psittaci genomes reveals the recent emergence of a pathogenic lineage with a broad host range. mBio 2013;4:e00604-12. [Crossref] [PubMed]
- Harkinezhad T, Verminnen K, Van Droogenbroeck C, et al. Chlamydophila psittaci genotype E/B transmission from African grey parrots to humans. J Med Microbiol 2007;56:1097-100. [Crossref] [PubMed]
- Heddema ER, van Hannen EJ, Duim B, et al. An outbreak of psittacosis due to Chlamydophila psittaci genotype A in a veterinary teaching hospital. J Med Microbiol 2006;55:1571-5. [Crossref] [PubMed]
- Suga T, Sugiyama Y, Fujii T, et al. Bronchioloalveolar carcinoma with bronchorrhoea treated with erythromycin. Eur Respir J 1994;7:2249-51. [Crossref] [PubMed]
- Zuzek R, Green M, May S. Severe psittacosis progressing to suspected organizing pneumonia and the role of corticosteroids. Respir Med Case Rep 2021;34:101486. [Crossref] [PubMed]
- Price ME, Harrison BD. Restrictive pattern of lung function following psittacosis treated with corticosteroids. Br J Dis Chest 1982;76:199-201. [Crossref] [PubMed]
- Hirata M, Noto M, Oda K, et al. A case of psittacosis presenting as adult respiratory distress syndrome and successfully treated with steroid pulse therapy. Kokyu To Junkan 1988;36:893-7. [PubMed]
- Dequin PF, Meziani F, Quenot JP, et al. Hydrocortisone in Severe Community-Acquired Pneumonia. N Engl J Med 2023;388:1931-41. [Crossref] [PubMed]
(English Language Editor: J. Jones)
Cite this article as: Huang C, Liu M, Gu B, Yuan C, Wang Y, Cai T, Gao J, Yin J, Yu S, Zhou M. Chlamydia psittaci causing severe pneumonia with an initial complaint of massive watery sputum: a case report. AME Case Rep 2024;8:27.


