
Concurrent chronic myelomonocytic leukemia and gastric carcinoma: a case report and literature review
Highlight box
Key findings
• CMML can be complicated by the presence of gastrointestinal solid tumors. In cases where patients are at high risk for gastrointestinal endoscopy, capsule endoscopy serves as a safer method for evaluating whether the patient requires additional endoscopic biopsy.
What is known and what is new?
• The coexistence of CMML and solid neoplasms in the same patient is exceptionally uncommon.
• When RBC infusion proves ineffective in CMML patients, it is crucial to consider the possibility of gastrointestinal malignant disease.
What is the implication, and what should change now?
• Further investigation is needed to explore the mechanisms underlying the association between CMML and other solid tumors.
Introduction
Chronic myelomonocytic leukemia (CMML) is a rare and aggressive myeloid neoplasm with myelodysplastic and myeloproliferative features. Its overall incidence ranges from 3.5 to 5.2 per million in the USA and Europe, and the rate increases significantly with age (1-3). CMML mainly occurs in the elderly patients aged 65–75 years, with a median overall survival (OS) of 12 months (3,4). The diagnostic criteria for CMML are as follows (5): (I) Prerequisite criteria: (i) persistent absolute (≥0.5×109/L) and relative (≥10%) peripheral blood monocytosis; (ii) blasts constitute <20% in the peripheral blood and bone marrow; (iii) did not meet the diagnostic criteria of chronic myeloid leukemia or other myeloproliferative neoplasms (MPNs); (iv) did not meet the diagnostic criteria of myeloid/lymphoid neoplasms with tyrosine kinase fusions. (II) Supporting criteria: (i) dysplasia involving ≥1 myeloid lineages; (ii) acquired clonal cytogenetic or molecular abnormality. (III) Requirements for diagnosis: (i) prerequisite criteria must be present in all cases; (ii) if monocytosis is ≥1×109/L, one or more supporting criteria must be met.
The diagnostic process of CMML can be challenging because the clinical presentation varies depending on whether the patient has myelodysplastic abnormalities or myelodysplastic features. The coexistence of CMML and solid neoplasms is extremely rare (6-8). Here, we report the case of a CMML patient who was diagnosed with gastric cancer concurrently. This case report is presented following the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-12/rc).
Case presentation
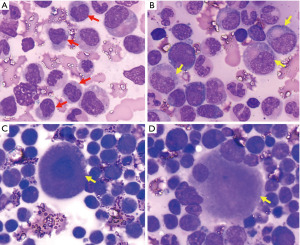
A 75-year-old Chinese woman was admitted to our hospital due to a one-month history of leukocytosis and anemia. Physical examination showed splenomegaly, but no palpable superficial lymphadenopathy. The spleen was 160 mm × 60 mm in size as seen in the abdominal ultrasonography. The complete blood count revealed white blood cells (WBC) 121.7×109/L with monocytes 70.68×109/L (58.1%) and neutrophils 44.12×109/L (36.2%), hemoglobin 59 g/L, hematocrit (HCT) 18.4%, mean corpuscular volume (MCV) 90.6 fL, mean corpuscular hemoglobin (MCH) 29.1 pg, mean corpuscular hemoglobin concentration (MCHC) 321 g/L and platelet 71×109/L. Bone marrow aspiration (Figure 1) revealed 2% blasts (2%) and 10.6% monocytes with dysplasia of myelocytes and megakaryocyte. Flow cytometric immunophenotyping indicated a positive test for CD13, CD14, CD33, CD36, HLA-DR, and CD56. BCR-ABL1 fusion gene and other genetic abnormalities (such as PDGFRA and PDGFRB rearrangements; JAK2, MPL, and CALR mutations, and others) were not detected, but CBL (p.C416S) and TET2 (p.L699* and p.R1261C) mutations were. karyotype was normal. Based on these results, the patient was diagnosed with myelodysplastic CMML (CMML-1). CPSS-Mol was two points, which was considered indicative of intermediate-2 risk.
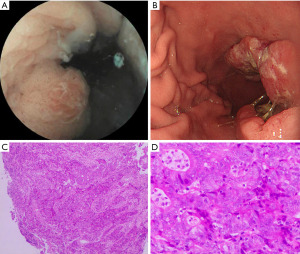
However, before CMML treatment, she had melena and an ineffective red blood cell (RBC) transfusion. A fecal occult blood test was positive. Tumor markers such as carcinoembryonic antigen (CEA) and coagulation function were all within the normal range. Moreover, the patient had no history of hemorrhagic diseases, such as liver disease, surgery, and use of anticoagulant and antiplatelet drugs. Due to the patient’s history of coronary heart disease, and because it had been less than half a year since her percutaneous coronary stenting, she underwent capsule endoscopy and abdominal enhanced computed tomography (CT) and three-dimensional reconstruction to initially assess whether there were space-occupying lesions in the gastrointestinal tract. The examination results suggested that there were space-occupying lesions in the stomach (Figure 2A). Further positron emission tomography/CT examination showed that the gastric antrum wall was significantly thickened, and that metabolic activity was significantly enhanced. After a multi-disciplinary team (MDT) discussion among the departments of cardiology, anesthesiology, and gastroenterology, the patient underwent painless gastroscopy and the tissue of the gastric lesion was taken for biopsy (Figure 2B-2D). Histopathologic biopsy suggested a moderately to poorly differentiated adenocarcinoma. Immunohistochemistry (IHC) were CK (+), CEA (+), HER-2 (−), P53 (70%, 2+), Ki67 (98%+), PMS2 (−), MLH1 (dim), MSH2 (+), and MSH6 (+). Hence, this patient was diagnosed with CMML with gastric adenocarcinoma. After pre-treatment with oral hydroxyurea, a total of six cycles of intravenous decitabine (10 mg/d × 7 d) for the CMML were administered every four weeks. She was also given 2.5 g of capecitabine per day (14 days in total) for the gastric adenocarcinoma every three weeks. However, these treatments did not elevate the low hemoglobin level and the patient died of sudden massive bleeding from the stomach.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s daughter after her death for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CMML is a rare hematopoietic disorder. It mainly affects elderly patients with a median age of 65–75 years. It is more common among white people than blacks, Asians or Pacific Islanders. Overall, 10–20% of patients with CMML develop acute myeloid leukemia (AML), which has poor prognosis (3,9). Before diagnosis of CMML, it is important to exclude reactive causes (infectious etiologies or connective tissue disorders) of monocytosis. The patient came to our hospital with a pulmonary infection and a mild elevation of C-reactive protein (CRP, 59 mg/L). After antibiotic treatment, computed tomography (CT) scan of lungs and CRP returned to normal, but monocyte levels remained high. The final diagnosis of CMML was based on peripheral blood and bone marrow examination.
Since the patient had a history of coronary heart disease, and since it had been less than half a year after percutaneous coronary stenting, the risk of gastrointestinal endoscopy was high. For this reason, the patient underwent capsule endoscopy and abdominal enhanced CT instead. Space-occupying lesions of the stomach were found following these examinations. Finally, the patient was diagnosed with gastric carcinoma. The patient had no risk factors for gastric carcinoma such as diet, smoking, alcohol consumption, or H. pylori (HP) infection. In human medicine, capsule endoscopes are used for several sets of indications. It was originally approved for the evaluation of patients with gastrointestinal bleeding of unknown cause, and it is widely used for this purpose as well as for other gastrointestinal diseases (10). Zeng et al. reported a case of Dieulafoy’s disease with gastric Mucosal-associated lymphoid tissue (MALT) lymphoma and found a large hemorrhage in the stomach under capsule endoscopy (11). Although producing excellent images of the upper gastrointestinal tract is challenging due to the capacious stomach and the rapid transition through the esophagus and duodenum, capsule endoscopy is a non-invasive approach for patients who are at high risk of gastrointestinal endoscopy, and it can identify patients who need conventional endoscopy for biopsy (12).
To our knowledge, coexistence of CMML and solid neoplasms in the same patient is very rare. In 1986, Sans-Sabrafen et al. (13) were the first authors to report five patients with CMML and epithelial tumors (two cases of stomach carcinoma, one case of colon carcinoma, one case of epidermoid bronchogenic carcinoma, and one case of an epidermoid tumor of unknown origin). Among them, three patients had CMML and an epithelial tumor simultaneously. The other two patients had tumors (epidermoid metastatic carcinoma and colon carcinoma) before getting CMML (13). However, the characteristics of these patients were not described in detail in this paper. Likewise, cases of CMML following other solid neoplasms (such as endometrial adenocarcinoma, cutaneous tumor, bladder papilloma, sigma carcinoma, and cutaneous T-cell lymphoma) have been reported (6,8). In our case, CMML and gastric carcinoma almost simultaneously occurred in this patient.
Due to the rarity of CMML, the mechanisms by which it is associated with other solid tumors are unknown. Some hypotheses have been proposed. Sans-Sabrafen et al. and Rovira et al. suggested that myelodysplastic syndrome (MDS) was a paraneoplastic manifestation of the solid tumor in some patients who had solid neoplasms and had not been treated previously by chemotherapy or radiation, but a coincidental association between the two should be considered (6,13). For those patients who had solid neoplasms prior to CMML and received chemotherapy, therapy-related leukemia should be considered. The most common chemotherapeutic agents that cause hematological malignancies (MDS or AML) are alkylating agents (such as cyclophosphamide and melphalan) and topoisomerase II agents (such as etoposide). For the first group of agents, malignancy occurs years after exposure with a latency of several years. For the second group of agents, malignancy occurs earlier after exposure with no latency (14). The patient in our case was diagnosed with CMML and gastric carcinoma at almost the same time. CMML may be a paraneoplastic manifestation of the solid tumor for this patient. However, Somatic mutations in TET2 and CBL in this patient revealed the clonal hematopoietic properties of CMML. So coincidental association should also be considered.
Treatment options for CMML patients are mainly based on risk stratification and symptoms. For low-risk patients, recommended approaches include observation and symptom-oriented treatment. For those at intermediate or high risk), bone marrow transplant evaluation should be considered. However, due to the lack of clinical trial evidence, determining the optimal treatment for CMML remains challenging. Allogeneic stem cell transplantation (ASCT) may remain the only potential cure for CMML (15). Unfortunately, the median age at diagnosis of CMML is approximately 70 years, which is why many patients are not transplant candidates. The drugs commonly used in CMML mainly include hypomethylation agents (HMAs, such as decitabine), hydroxyurea and erythropoietin stimulating agents (ESAs). The overall response rate of decitabine in the treatment of advanced CMML has been reported to be 38%, in which the TET2 mutation did not affect the response or survival rate of CMML patients receiving decitabine (16). However, HMAs have not been shown to alter the natural course and prognosis of CMML (15,17). There are many promising new drugs on the horizon, including luspatercep, lenzilumab and tagraxofusp, and the results are encouraging (18-20). Therefore, when treatment is indicated, clinical trials should be considered.
Mutations of genes such as ASXL1, NRAS, SETBP1, RUNX1, CBL, DNMT3A, SRSF2, and EZH2 have been shown to be associated with poor prognosis (21-26). However, among all models that consider molecular information [Groupe Français des Myélodysplasies (GFM), Mayo Molecular Model (MMM), and CMML-specific Prognostic Scoring System molecular score (CPSS-Mol)], only ASXL1 is consistently included. A consensus model is needed to provide uniform, standardized risk stratification and treatment for patients with CMML. Additionally, further genetic studies of these mutations are needed in the future to determine whether they are suitable therapeutic targets.
Conclusions
This report describes a rare case of CMML with gastric cancer. It highlights the need for clinical suspicion of complicated gastrointestinal malignant diseases in addition to primary disease when RBC infusion is ineffective in CMML patients. For patients who are at high risk for gastrointestinal endoscopy, capsule endoscopy may be a safer approach to determining whether the patient needs further conventional endoscopy for biopsy. The mechanism of CMML with solid tumor, treatment and prognosis of CMML are also reviewed. There are currently no treatment guidelines for patients with CMML and gastric carcinoma. Personalized treatment is recommended.
Acknowledgments
Funding: This research was supported by Chinese National Major Project for New Drug Innovation (No. 2019ZX09201002003), National Natural Science Foundation of China (Nos. 82030076, 82070161, 81970151, 81670162 and 81870134), Shenzhen Science and Technology Foundation (Nos. JCYJ20190808163601776 and JCYJ20200109113810154), Shenzhen Key Laboratory Foundation (No. ZDSYS20200811143757022), Sanming Project of Medicine in Shenzhen (No. SZSM202111004), Natural Science Foundation of Shenzhen University General Hospital (No. SUGH2019QD012), and Shenzhen Natural Science Fund (the Stable Support Plan Program, No. 20200830182623001).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-12/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-12/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s daughter after her death for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Phekoo KJ, Richards MA, Møller H, et al. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica 2006;91:1400-4. [PubMed]
- Maynadié M, Girodon F, Manivet-Janoray I, et al. Twenty-five years of epidemiological recording on myeloid malignancies: data from the specialized registry of hematologic malignancies of Cote d'Or (Burgundy, France). Haematologica 2011;96:55-61. [Crossref] [PubMed]
- Guru Murthy GS, Dhakal I, Mehta P. Incidence and survival outcomes of chronic myelomonocytic leukemia in the United States. Leuk Lymphoma 2017;58:1648-54. [Crossref] [PubMed]
- Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2016 update on diagnosis, risk stratification, and management. Am J Hematol 2016;91:631-42. [Crossref] [PubMed]
- Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022;36:1703-19.
- Rovira M, Cervantes F, Lozano M, et al. Chronic myelomonocytic leukemia and solid neoplasms: is it a causal or a fortuitous association? angre (Barc) 1989;34:207-9.
- Fernández-Escribano M, Junquera JM, Santos I. Chronic myelomonocytic leukemia and renal carcinoma: a rare association. Sangre (Barc) 1997;42:88-9. [PubMed]
- Yoshimi A, Asai T, Maeda D, et al. Chronic myelomonocytic leukemia presenting severe uterine hemorrhage due to uterine infiltration of leukemic cells and early-stage endometrial adenocarcinoma. Arch Gynecol Obstet 2009;280:1077-8. [Crossref] [PubMed]
- Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 2002;99:840-9. [Crossref] [PubMed]
- Nakamura T, Terano A. Capsule endoscopy: past, present, and future. J Gastroenterol 2008;43:93-9. [Crossref] [PubMed]
- Zeng Q, Dai JF, Cao H, et al. Dieulafoy disease with gastric MALT lymphoma: A case report. Medicine (Baltimore) 2020;99:e22651. [Crossref] [PubMed]
- Tai FWD, Ching HL, Hale MF, et al. Upper gastrointestinal endoscopy: can we cut the cord? Lancet Gastroenterol Hepatol 2019;4:749-51. [Crossref] [PubMed]
- Sans-Sabrafen J, Woessner S, Besses C, et al. Association of chronic myelomonocytic leukemia and carcinoma: a possible paraneoplastic myelodysplasia. Am J Hematol 1986;22:109-10. [Crossref] [PubMed]
- Ahmed F, Osman N, Lucas F, et al. Therapy related CMML: a case report and review of the literature. Int J Hematol 2009;89:699-703. [Crossref] [PubMed]
- Chan O, Renneville A, Padron E. Chronic myelomonocytic leukemia diagnosis and management. Leukemia 2021;35:1552-62. [Crossref] [PubMed]
- Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood 2011;118:3824-31. [Crossref] [PubMed]
- Merlevede J, Droin N, Qin T, et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat Commun 2016;7:10767. [Crossref] [PubMed]
- Platzbecker U, Germing U, Götze KS, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol 2017;18:1338-47. [Crossref] [PubMed]
- Patnaik MM, Sallman DA, Mangaonkar AA, et al. Phase 1 study of lenzilumab, a recombinant anti-human GM-CSF antibody, for chronic myelomonocytic leukemia. Blood 2020;136:909-13. [Crossref] [PubMed]
- Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N Engl J Med 2019;380:1628-37. [Crossref] [PubMed]
- Elena C, Gallì A, Such E, et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016;128:1408-17. [Crossref] [PubMed]
- Patnaik MM, Barraco D, Lasho TL, et al. DNMT3A mutations are associated with inferior overall and leukemia-free survival in chronic myelomonocytic leukemia. Am J Hematol 2017;92:56-61. [Crossref] [PubMed]
- Padron E, Garcia-Manero G, Patnaik MM, et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 2015;5:e333. [Crossref] [PubMed]
- Patnaik MM, Vallapureddy R, Lasho TL, et al. EZH2 mutations in chronic myelomonocytic leukemia cluster with ASXL1 mutations and their co-occurrence is prognostically detrimental. Blood Cancer J 2018;8:12. [Crossref] [PubMed]
- Damm F, Itzykson R, Kosmider O, et al. SETBP1 mutations in 658 patients with myelodysplastic syndromes, chronic myelomonocytic leukemia and secondary acute myeloid leukemias. Leukemia 2013;27:1401-3. [Crossref] [PubMed]
- Grossmann V, Kohlmann A, Eder C, et al. Molecular profiling of chronic myelomonocytic leukemia reveals diverse mutations in >80% of patients with TET2 and EZH2 being of high prognostic relevance. Leukemia 2011;25:877-9. [Crossref] [PubMed]
Cite this article as: Fang C, Chen Z, Li J, Yu L, Wang L. Concurrent chronic myelomonocytic leukemia and gastric carcinoma: a case report and literature review. AME Case Rep 2023;7:37.


