Acquired perforating dermatosis in patients on peritoneal dialysis: a report of 3 cases
Highlight box
Key findings
• Acquired perforating dermatosis (APD) is more commonly observed in patients with chronic kidney disease (CKD) and concomitant conditions such as diabetes mellitus (DM).
• Poor control of calcium and phosphorus metabolism is associated with the development of APD in CKD patients.
• This study sheds light on the underreported occurrence of APD in patients undergoing peritoneal dialysis (PD).
What is known and what is new?
• APD is a rare condition characterized by umbilicated papules, itching, and epidermal perforation.
• Previous research has documented the association of APD with CKD and DM, but our study specifically focuses on APD in PD patients, filling a knowledge gap.
Implications and recommendations
• Healthcare professionals should be vigilant for APD in CKD and PD patients, considering dermatological screenings.
• Optimal control of calcium and phosphorus metabolism may play a role in preventing or mitigating APD development.
• Collaboration between nephrologists and dermatologists is vital for improved APD care.
Introduction
Acquired perforating dermatosis (APD) is a heterogeneous group of unfrequented diseases (2.5 cases for 100,000 habitants) (1) characterized by the formation of multiple-size itchy papules or plaques with a central keratotic core with the Koebner phenomenon. It usually affects the legs and extensor surfaces (2). Histologically, the epidermis becomes hyperplastic surrounding the dermal component, causing its transepidermal elimination following keratinocyte maturation. APD encompasses distinct subtypes based on the specific dermal component involved in the elimination process. These include elastosis perforans serpiginosa, where abnormal elastic tissue fibers are eliminated; reactive perforating collagenosis (RPC), which involves the elimination of altered collagen; Kyrle’s disease, characterized by the elimination of keratin; and perforating folliculitis, where the follicle content is removed, which may include collagen and elastic fibers (3).
The subtype ‘acquired’ refers to a perforating dermatosis usually beginning in adulthood, associated with multiple diseases, especially diabetes mellitus (DM) and end-stage renal disease (ESRD). A prevalence between 4–11% of hemodialysis patients has been noticed, being less common in patients on peritoneal dialysis (PD) (4-6). Here we described 3 cases of APD in patients on PD, one of them with a giant variant of RPC. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-9/rc).
Case presentation
Case 1
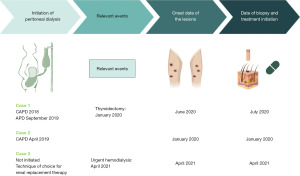
A 28-year-old man with chronic kidney disease (CKD) secondary to a Dent’s disease initiated continuous ambulatory peritoneal dialysis (CAPD) in 2018; with an initial correct peritoneal dialysis adequacy, he needed to change to automated peritoneal dialysis, 9-hour schedule with 5 exchanges of 2,000 cc of 2.27% and 1.36% glucose solutions, after a bilateral nephrectomy in September 2019. Despite the excellent adequacy (KT/V 2.5), one of the problems to be emphasized was the lousy control of disturbances of calcium-phosphate metabolism with phosphorus levels above 6 mg/dL, parathyroid hormone (PTH) above 500 pg/mL and phosphor-calcium product over 55 mg2/dL2. Furthermore, in January 2020 was thyroidectomized because of a malign nodule.
In June 2020, he noticed the appearance of itchy keratotic papules on his legs and brought it to the attention of the doctor during an external consultation at the Nephrology Department. The nephrologist referred him to the Dermatology Department, where a biopsy was performed. The biopsy results revealed significant acanthosis and papillomatosis with the presence of ortho and parakeratotic horn plugs, along with keratin strands and some collagen fibers in the epidermis. Additionally, areas of elastic fibers penetrating the epidermis and stratum corneum were observed, consistent with APD. Treatment with isotretinoin at a dosage of 0.5 mg/kg of body weight was initiated, resulting in a favorable subsequent evolution.
Case 2
A 44-year-old man with hypertension, DM type 1, and CKD secondary to diabetic kidney disease (DKD), initiated CAPD in April 2019 with three daily exchanges of 1,500 cc each. Two exchanges with a concentration of 2.27% and one exchange with a concentration of 1.36% with optimal PD adequacy (December 2019: KT/V 2.5). Of note, he had poor control of disturbances of calcium-phosphate metabolism (December 2019: calcium: 8.7 mg/dL, phosphorus: 5.9 mg/dL, PTH: 360 pg/mL, 25-hydroxyvitamin D: 13.7 ng/mL).
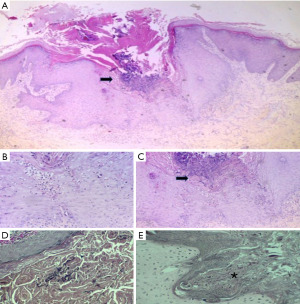
During a routine control in January 2020, he developed itchy keratotic papules on his legs. Additionally, his residual renal function was impaired, resulting in inadequate adequacy of peritoneal dialysis (KT/V 1.6). As a result, the number of peritoneal dialysis exchanges had to be increased, and he was referred to the Dermatology service for assessment of the lesions. A biopsy was performed, which revealed a central crateriform area filled with parakeratotic keratin and debris, along with a mild inflammatory infiltrate consisting of intraepidermal polymorphonuclear leukocytes. Beneath this area, there was evidence of transepidermal migration of collagen fibers, while no elastic fibers were observed. These findings are consistent with RPC (Figure 1). After the diagnosis, the Dermatologist initiated treatment for the patient using isotretinoin at a dosage of 0.5 mg/kg of body weight and heliotherapy. The patient responded well to the treatment, showing positive progress in their condition.
Case 3
A 58-year-old man with DM type 2, obesity, rheumatoid arthritis, hypothyroidism, and CKD secondary to DKD. His estimated glomerular filtration rate (eGFR) was 13 mL/min, and he was being monitored by the advanced CKD Unit. PD was chosen as the preferred renal replacement therapy method. One of the challenges in his management was the inadequate control of mineral bone metabolism, characterized by elevated phosphorus levels above 7 mg/dL and PTH levels exceeding 400 pg/mL.
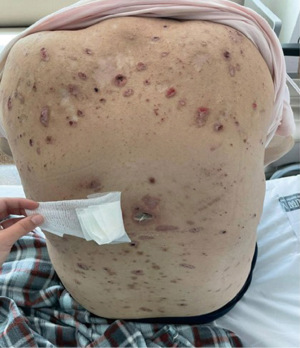
In April 2021, the patient experienced an acute decline in renal function, necessitating the initiation of urgent hemodialysis. Concurrently, he developed extensive, keratotic, and highly pruritic skin lesions on his back, with some exceeding 2 cm in size. Furthermore, certain lesions became infected due to scratching (Figure 2).
The Dermatology Department suspected that the lesions were infected due to scratching. However, a biopsy was conducted, revealing a flattened epidermis with significant ulceration, fibrin deposits, cellular debris, and an acute inflammatory response. At the level of the dermal-epidermal junction, vertically oriented collagen fibers were observed, projecting towards the surface. These histopathological findings are consistent with RPC. Considering the lesion size exceeding two centimeters, the diagnosis of a giant variant of RPC was established.
Corticosteroid therapy, isotretinoin at a dosage of 0.5 mg/kg of body weight, and phototherapy were initiated in an attempt to manage the condition. However, despite these interventions, the patient did not show optimal improvement in their condition. Unfortunately, the patient passed away in October 2021 due to cardiovascular causes.
Historical and current information from the three cases has been organized in a timeline format, which is presented in Figure 3.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
APD are a group of uncommon conditions characterized by umbilicated papules accompanied by a central keratotic cap and widespread itching. The most commonly affected areas include the trunk and limbs, which aligns with the presentation observed in our patients (2).
Histologically, APD are characterized by a central keratotic plug that overlies and area of epidermal perforation. This perforation allows for the transepidermal elimination of one or more dermal components, leading to the identification of any of the four classic perforating diseases: elastosis perforans serpiginosa, RPC, Kyrle’s disease, and perforating folliculitis. It is important to note that, similar to our first case, multiple patters of APD may be observed within a single patient (3).
The association of APD with multiple systemic diseases has been well-documented (Figure 4). Among the various associations observed in case series, one of the most common in the coexistence of DM an CKD, primarily caused by DKD (6,7).
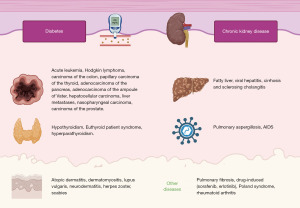
In our case series, two patients had DM and CKD secondary to DKD. Additionally, two patients had thyroid disorders, with the first case resulting from a thyroidectomy secondary to papillary carcinoma of the thyroid and the third case due to hypothyroidism. Notably, the third case also presented with rheumatoid arthritis, which, although less frequent, has been reported in five patients in the literature (8). It is important to highlight that the final case represents a giant variant of RPC, characterized by the substantial size of the lesions (9,10). Furthermore, this patient had the simultaneous presence of four concomitant diseases, including CKD secondary to DKD, DM, hypothyroidism, and rheumatoid arthritis.
Although APD can occur prior to renal replacement therapy, it is more commonly observed in dialysis patients. The prevalence of APD in hemodialysis patients has been reported to range from 4% to 11% and in the majority of cases reported in the literature, it is associated with hemodialysis rather than PD (4-7). In fact, in the largest series published to date by Saray et al., only 2 cases involved patients on PD, while 14 cases involved patients on hemodialysis, and 6 cases were observed in patients without any dialysis treatment (11).
In our case series, we present two patients who were undergoing PD and one patient who had not yet initiated dialysis but required urgent initiation. However, it should be noted that the relationship between APD and kidney disease cannot be solely attributed to its etiopathogenesis, as the onset of lesions has been associated with a decline in kidney function in some reported cases (7).
The exact pathogenesis of APD remains unclear, as it appears to involve multiple interacting factors. However, itching plays a crucial role in this condition. Scratching leads to microtrauma that alters the collagen fibers in the papillary dermis, facilitating their transepidermal elimination in the final stage of the disease process. Additionally, other contributing factors may include impaired wound healing capacity, tissue hypoxia induced by DM, microangiopathy, and oxidative stress associated with CKD (2).
It is noteworthy that all three patients in our case series exhibited poor control of phosphorus-calcium metabolism, which is known to be one of the primary causes of pruritus in dialysis patients (12). This association may explain the relationship between inadequate control of phosphorus-calcium metabolism and the development of APD.
There is limited information available in the literature regarding the association between APD and PD. However, a case study reported by González-Lara et al. described a patient who developed APD while undergoing PD, and showed improvement after switching to hemodialysis (7). This funding suggests a potential causal relationship between APD and PD itself.
One possible mechanism for this association is the use of glucose-based solutions in PD, which may contribute to the worsening of DM. Additionally, in combination with the presence of CKD, these factors may precipitate the development of APD. Nevertheless, further specific studies would be required to confirm this hypothesis.
First-line treatment options for APD include the use of retinoids, systemic of topical corticosteroids, and keratolytic agents. Oral emollients and antihistamines are commonly prescribed to alleviate itching. However, in cases where these approaches are insufficient, alternative treatment modalities may be necessary. There have been reports of successful outcomes with the use of tetracyclines, phototherapy, heliotherapy and allopurinol (13).
In our cases, all patients were treated with retinoids as the primary therapeutic approach. In the second case, heliotherapy was additionally employed, while in the most severe case, a combination of corticosteroids and phototherapy was utilized; however, these interventions did not yield successful outcomes.
Conclusions
In conclusion, CKD and associated conditions such as DM are associated with an increased risk of developing APD. Our cases highlight the frequent presence of poor control of calcium and phosphorus metabolism in CKD patients, with appears to be linked to the development of APD.
Through the presentation of these cases, we aim to emphasize the significance of recognizing this rare disease, particularly in patients undergoing PD, as it is less commonly reported in comparison to patients undergoing hemodialysis.
Acknowledgments
The authors thank the patients and their families, and medical research staff who participated in this case report.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-9/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- García-Malinis AJ, Del Valle Sánchez E, Sánchez-Salas MP, et al. Acquired perforating dermatosis: clinicopathological study of 31 cases, emphasizing pathogenesis and treatment. J Eur Acad Dermatol Venereol 2017;31:1757-63. [Crossref] [PubMed]
- Harbaoui S, Litaiem N. Acquired Perforating Dermatosis. 2021 Feb 23. In StatPearls. Treasure Island (FL): SteatPearls Publishing; 2021 Jan.
- Fernandes KA, Lima LA, Guedes JC, et al. Acquired perforating dermatosis in a patient with chronic renal failure. An Bras Dermatol 2016;91:10-3. [Crossref] [PubMed]
- Nickoloff BJ, Noodleman FR, Abel EA. Perforating pseudoxanthoma elasticum associated with chronic renal failure and hemodialysis. Arch Dermatol 1985;121:1321-2. [Crossref] [PubMed]
- Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol 1996;135:671-7. [Crossref] [PubMed]
- Imam TH, Patail H, Khan N, et al. Acquired Perforating Dermatosis in a Patient on Peritoneal Dialysis: A Case Report and Review of the Literature. Case Rep Nephrol 2018;2018:5953069. [Crossref] [PubMed]
- González-Lara L, Gómez-Bernal S, Vázquez-López F, et al. Dermatosis perforante adquirida: presentación de 8 casos. Actas Dermo-Sifiliograficas 2014;105:e1-5. [Crossref]
- Navarro-Rojas MM, Lemini-López A, Castellanos-Pallares G. Colagenosis perforante reactiva adquirida en una paciente con artritis reumatoide. Asociación poco frecuente. Dermatol Rev Mex 2021;65:S120-4.
- Kim RH, Kwa M, Adams S, et al. Giant acquired reactive perforating collagenosis in a patient with diabetes mellitus and metastatic breast carcinoma. JAAD Case Rep 2016;2:22-4. [Crossref] [PubMed]
- Hoque SR, Ameen M, Holden CA. Acquired reactive perforating collagenosis: four patients with a giant variant treated with allopurinol. Br J Dermatol 2006;154:759-62. [Crossref] [PubMed]
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol 2006;20:679-88. [Crossref] [PubMed]
- Santos-Alonso C, Maldonado Martin M, Sánchez Villanueva R, et al. Prurito en pacientes en diálisis. Revisión de la literatura y nuevas perspectivas. Nefrología 2022;42:15-21. [Crossref] [PubMed]
- Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges 2018;16:825-42. [Crossref]
Cite this article as: Viejo-Boyano I, López-Romero LC, Martínez-i-Cózar V, Soldevila-Orient A, Hernández-Jaras J. Acquired perforating dermatosis in patients on peritoneal dialysis: a report of 3 cases. AME Case Rep 2023;7:46.