
Conservative treatment of type A aortic dissection: a case report with 5 years of follow-up
Highlight box
Key findings
• In carefully selected non-operable patients, conservative treatment can effectively manage type A aortic dissection (TAAD), provided that strict adherence to therapy is ensured.
What is known and what is new?
• Emergent surgical intervention remains the treatment of choice for TAAD.
• Ensuring strict therapeutic adherence to conservative treatment may be crucial for attaining a successful outcome in non-operable patients with TAAD.
What is the implication, and what should change now?
• Conservative treatment can be a viable alternative to surgery for high-risk patients with TAAD.
• This case highlights the need to carefully consider individual patient factors and closely monitor therapeutic adherence to treatment protocols to optimize outcomes.
Introduction
Aortic dissection is an acute aortic pathology in which the luminal layers of the vessel progressively separate due to a tear in the tunica interna. The Stanford classification divides aortic dissections into two general types based on the lesion’s anatomical distribution (1). Specifically, dissections involving the ascending aorta, as it branches off the heart, are defined as type A aortic dissection (TAAD), while all other distributions are type B aortic dissection (TBAD). This division has direct therapeutic and prognostic implications since a substantial proportion of patients with TAAD die before reaching the hospital (approximately 60% with TAAD, and 20% with TBAD) (2). This is not surprising considering the proximity of TAAD to the aortic root, which is affected in two-thirds of patients dying acutely from the disease, usually secondary to hemorrhagic pericardial tamponade (3,4). Therefore, TAAD requires immediate surgical attention, while TBAD generally is more optimally managed through conservative efforts only (5). However, surgical intervention may be contraindicated in older patients and those with severe comorbidities. The latter patient category represents a significant clinical challenge since these patients are significantly more likely to develop aortic dissection. Indeed, the incidence of acute aortic disease in the general population increases from about 5–16 cases per 100,000 person-years to more than 30 in patients older than 65 years (6,7). Additionally, more than 70% of all patients with an aortic dissection present with a prior history of arterial hypertension (8). Moreover, factors related to the anatomical complexity of the ascending aorta challenge acute therapeutic management [e.g., via conventional surgery or thoracic endovascular ascending aortic repair (TEVAR)] and may increase procedure-related complications such as stroke, and cardiopulmonary dysfunction. The literature on long-term follow-up on medically managed TAAD is sparse. Herein, we report a case of an 86-year-old female with TAAD treated conservatively with long-term follow-up. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-107/rc).
Case presentation
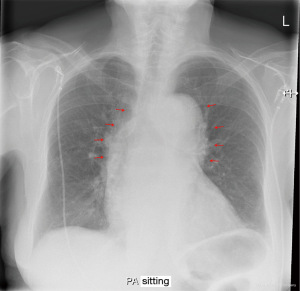
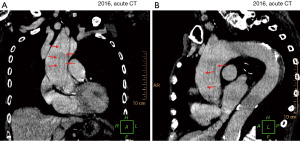
An 86-year-old Caucasian female with a family history of hypercoagulability and a past medical history of arterial hypertension, paroxysmal atrial fibrillation, polymyalgia, chronic pain syndrome, and previous pulmonary embolisms presented with acute central chest pain radiating to the neck and left shoulder. The pain had started suddenly during gardening work. The patient had a history of mild non-cardiac chest pain, which was attributed to dyspepsia. However, this time the pain rapidly intensified, making the patient short of breath and diaphoretic. Within 1 h, the patient was admitted to the emergency department at Randers Regional Hospital, Denmark. The patient was diaphoretic at admission, but the dyspnea had subsided. Moreover, the patient was conscious and without any neurological symptoms. Cardiac auscultation identified a systolic murmur, which was known from previous admissions. Blood pressure was 160/88 mmHg, and the initial electrocardiogram (ECG) showed slight sinus bradyarrhythmia with right bundle branch block (unchanged from past ECG’s). Subsequent telemetric heart rate monitoring revealed a heart rate frequency ranging between 60 and 110 bpm. Myocardial biochemical markers were within normal ranges. A chest X-ray demonstrated a widened 11 cm mediastinal silhouette (Figure 1). Transthoracic echocardiography (TTE) showed no pericardial effusion, no heart valve involvement, and normal left ventricular, hence no signs of myocardial malperfusion were detected. Subsequent acute computed tomography (CT) imaging revealed a massive (type A4 SVS/STS, Debakey type 1a) aortic dissection with an entry tear 3 cm above the aortic valve. Distal extent 4 cm into the descending arc with branch extensions in the brachiocephalic trunk and the right common carotid artery. The false lumen was patent with a cross-sectional size of 26 mm, while the true lumen was 15 mm (Figure 2, Video 1). No radiologic signs of malperfusion syndrome were detected.
The patient had an international normalized ratio (INR) of 3.6 upon presentation, likely due to habitual anticoagulant medication (warfarin), which had been initiated prophylactically due to the estimated thromboembolic risk associated with atrial fibrillation and prior pulmonary emboli (CHA2DS-VASc =6). Upon discovering the aortic dissection, the medication was promptly stopped and reversed with phytomenadione to restore a procoagulant effect.
The patient had a clinical frailty score (CFS) of 6, indicating moderate frailty. The patient’s pulmonary function, with arterial blood gas and pulse oximetry, was normal, and renal function was within the normal range (plasma-creatinine ~0.8 mg/dL).
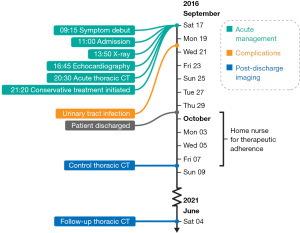
Considering the patient’s age, comorbidities, and the extent of the aortic dissection, the cardiothoracic team predicted the surgical risk to be prohibitively high. Moreover, the patient preferred conservative treatment over surgery. An antihypertensive regimen comprising labetalol, a thiazide diuretic, and nitroglycerin was initiated resulting in a systolic blood pressure of around 120 mmHg. By the end of the first week, the patient was transitioned to a long-term blood pressure control regime by the gradual replacement of labetalol and nitroglycerin with ramipril and amlodipine. The patient’s biochemical profile remained near habitual throughout the entire admission with a plasma creatinine level of around 0.8 mg/dL and plasma hemoglobin near 11.3 g/dL. Besides a minor urinary tract infection treated with pivmecillinam, the patient had an uneventful recovery and was discharged 13 days after admission. No repeat CT imaging was made prior to discharge because any subacute CT findings would be management inconsequential due to the patient’s surgical noncandidacy. Instead, it was decided that repeat CT imaging and outpatient control would be performed 1-month post-discharge. In the meantime, a home nurse monitored the patient’s blood pressure once daily and administered labetalol as necessary to keep systolic pressure below the target of 120 mmHg. An overview of the timeline is provided in Figure 3.
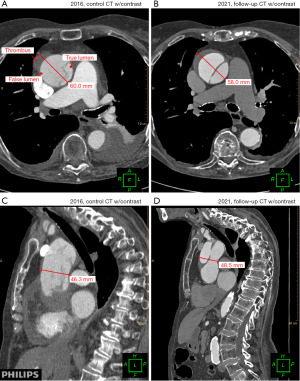
Pleural effusion was detected on the 1-month CT control scan and subsequently pleuracentesis was performed. The CT scan revealed a slight proximal progression of the aortic dissection and thrombosis of the false aortic lumen, but no expansion of luminal size.
Five years after the initial diagnosis, the patient presented again to the emergency department with radiating chest pain. Still, cardiac biomarkers were negative, and the ECG was unchanged. A CT scan found that the dissection had further thrombosed with a slight narrowing of the false lumen and a 2-mm distension of the aortic trunk (Figure 4, Video 2). Notably, the extent of the aortic dissection was unchanged since the 1-month follow-up. Now, more than 5 years after the initial presentation, the patient remains self-reliant and mobile despite having an extensive, yet stable, TAAD at age 92 years.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The treatment of choice for ascending aortic aneurysms or dissections is open surgical repair requiring sternotomy, cardiopulmonary bypass, deep hypothermic circulatory arrest, and selective cerebral perfusion (9). However, the surgical risk may be prohibitively high, e.g., due to substantial comorbidities, old age, anatomical complexities, or previous cardiac procedures. Such patients may benefit from an alternative approach, e.g., TEVAR (10), or conservative treatment (11). Although there have been great innovations in operative techniques, procedural strategies, and periprocedural intensive care management during the last decades, the 30-day mortality due to TAAD remains high, estimated at about 5–25% in high-volume centers (12). Moreover, major complications, e.g., stroke, renal failure, prolonged ventilation requirement, and reoperation, occur in 5–10% of patients after elective conventional repair (12).
The risk of mortality from acute aortic dissections increases significantly with patient age and risk factors, such as a prior history of heart surgery, hypertension, and anticoagulative status (13,14). Indeed, the patient, in this case, had arterial hypertension and adhered to anticoagulant therapy. More broadly, the surgical candidacy of octogenarians for TAADs has long been a point of contention. A study based on a single-center sample showed that aortic repair in octogenarians compared favorably with patients aged 30–65 years (15). The authors, therefore, argued that patient age should not be an obstacle to recommending surgery. Contrasting with this argument, a recent retrospective multicenter study argued for conservative measures, reporting that emergent surgery in octogenarians with an AAD carries an excessively high risk of intraoperative mortality while producing no statistically significant difference in the long-term survival compared to medically treated patients (16).
This ongoing debate centers on the statistic that patients with TAAD who do not undergo surgical treatment are more likely to die acutely than patients who undergo surgery (2). It has been argued that the increased mortality of TAAD is due to the lesion’s proximity to the cardioaortic root and, consequently, the risk of hemorrhagic pericardial tamponade and/or coronary involvement with acute myocardial ischemia, the principal causes of death in these patients (4). Indeed, most patients that present with tamponade and are conservatively treated die during initial hospitalization (17). In line with this argument, the patient in this study presented with no echocardiographic signs of valve involvement, lowering the a priori risk of mortality.
During the acute phase of the disease, the dissective process is highly volatile due to high vascular flow and shear stress from (reflex) hypertension not yet medically addressed (18,19), and a false lumen not yet fully blocked by thrombosis (20). Accordingly, data shows that the acute phase of TAAD accounts for the majority of the disease’s cumulative mortality and that by surviving past this critical phase, the natural history of the pathology begins to resemble that of the less lethal type B counterpart (16).
This merits several points of consideration for the acute treatment of aortic dissections. First, the duration of time since debut plays a major role in the treatment’s risk assessment: considering the 1–3% per hour mortality rate of TAAD, the a posteriori risk of a fatal outcome falls dramatically as the time with no major complications increases (21). Indeed, in the case presented here, the patient had already survived 12–24 hours since presenting with symptoms, which may have reduced her risk for acute mortality. Likewise, the initial echocardiographic assessment detected no valvular involvement or cardiac malperfusion, further reducing the risk. Despite the possibility of retrograde expansion (22), these factors, including a small aortic cross-section, favor a more low-risk, conservative treatment strategy for containing the dissection than surgery (23,24). Second, as has previously been noted (6,25), the effectiveness of conservative treatment to contain the dissection hinges on early and aggressive antihypertensive (“anti-impulse”) therapy to reduce shear stress. For the same reason, the ability of the blood to coagulate should be normalized to facilitate thrombosis of the dissection’s false lumen, thus blocking vascular flow from entering. Procoagulant therapy should, however, be cautioned due to the risk of adversities, such as cardioembolic events. Prophylactic measures using low-molecular heparins are recommended. Third, having the patient’s state daily controlled by a home nurse allowed for careful adherence to the therapeutic protocol, which may have facilitated a good outcome. Accordingly, a number of studies have recently shown that poor medication adherence is a frequent cause of treatment failure in patients with aortic dissection (26,27).
Conclusions
Octogenarians and nonagenarians with TAAD and severe comorbidities may be treatable through conservative intervention. This case report described the successful long-term conservative management of TAAD in an 86-year-old female with significant comorbidities that disqualified her as a surgical candidate. This lends support to acute risk stratification in TAAD patients before management decision-making according to, e.g., time since debut, the presence of pericardial tamponade, or coronary artery involvement. Medical management of ascending aortic dissection is an alternative approach in selected very high-risk patients. Importantly, therapeutic adherence should be maximized, e.g., through continuous monitoring, to optimize patient outcomes. More studies are needed.
Acknowledgments
The authors thank the departments and the 92-year-old patient who consented to the publication of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-107/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-22-107/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-107/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Yamaguchi T, Nakai M, Yano T, et al. Population-based incidence and outcomes of acute aortic dissection in Japan. Eur Heart J Acute Cardiovasc Care 2021;10:701-9. [Crossref] [PubMed]
- Isselbacher EM, Cigarroa JE, Eagle KA. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation 1994;90:2375-8. [Crossref] [PubMed]
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200-6. [Crossref] [PubMed]
- Zhu Y, Lingala B, Baiocchi M, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol 2020;76:1703-13. [Crossref] [PubMed]
- Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly 2017;147:w14489. [Crossref] [PubMed]
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Oderich GS, Pochettino A, Mendes BC, et al. Endovascular Repair of Saccular Ascending Aortic Aneurysm After Orthotopic Heart Transplantation Using an Investigational Zenith Ascend Stent-Graft. J Endovasc Ther 2015;22:650-4. [Crossref] [PubMed]
- Takayama H, Salerno CT, Aldea GS, et al. Characteristics of extracoronary vascular disease in heart transplant recipient. J Card Surg 2008;23:459-63. [Crossref] [PubMed]
- Salhab K, Gioia W, Rabenstein AP, et al. Medical Management of Three Patients with an Acute Type A Aortic Dissection: Case Series and a Review of the Literature. Aorta (Stamford) 2018;6:98-101. [Crossref] [PubMed]
- Tanaka K, Chikazawa G, Sakaguchi T, et al. Hybrid treatment for type A acute aortic dissection with multiorgan malperfusion. Ann Thorac Surg 2014;98:1118-20. [Crossref] [PubMed]
- Centofanti P, Flocco R, Ceresa F, et al. Is surgery always mandatory for type A aortic dissection? Ann Thorac Surg 2006;82:1658-63; discussion 1664. [Crossref] [PubMed]
- Sromicki J, Van Hemelrijck M, Schmiady MO, et al. Prior intake of new oral anticoagulants adversely affects outcome following surgery for acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2022;35:ivac037.
- Tang GH, Malekan R, Yu CJ, et al. Surgery for acute type A aortic dissection in octogenarians is justified. J Thorac Cardiovasc Surg 2013;145:S186-90. [Crossref] [PubMed]
- Dumfarth J, Peterss S, Luehr M, et al. Acute type A dissection in octogenarians: does emergency surgery impact in-hospital outcome or long-term survival? Eur J Cardiothorac Surg 2017;51:472-7. [Crossref] [PubMed]
- Gilon D, Mehta RH, Oh JK, et al. Characteristics and in-hospital outcomes of patients with cardiac tamponade complicating type A acute aortic dissection. Am J Cardiol 2009;103:1029-31. [Crossref] [PubMed]
- Khoynezhad A, Plestis KA. Managing emergency hypertension in aortic dissection and aortic aneurysm surgery. J Card Surg 2006;21:S3-7. [Crossref] [PubMed]
- Varon J, Marik PE. The diagnosis and management of hypertensive crises. Chest 2000;118:214-27. [Crossref] [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59. [Crossref] [PubMed]
- Wong DR, Lemaire SA, Coselli JS. Managing dissections of the thoracic aorta. Am Surg 2008;74:364-80. [Crossref] [PubMed]
- Ruan Y, Wang Z, Wu Z, et al. Painless retrograde type A aortic dissection followed conservative treatment of type B aortic dissection: a case report. BMC Cardiovasc Disord 2020;20:17. [Crossref] [PubMed]
- Kozai Y, Watanabe S, Yonezawa M, et al. Long-term prognosis of acute aortic dissection with medical treatment: a survey of 263 unoperated patients. Jpn Circ J 2001;65:359-63. [Crossref] [PubMed]
- Shingu Y, Myojin K, Ishibashi Y, et al. Is conservative therapy acceptable for thrombosed type A acute aortic dissection? Jpn J Thorac Cardiovasc Surg 2003;51:496-9. [Crossref] [PubMed]
- Feldman M, Shah M, Elefteriades JA. Medical management of acute type A aortic dissection. Ann Thorac Cardiovasc Surg 2009;15:286-93. [PubMed]
- Chaddha A, Erickson S, Kline-Rogers E, et al. Medication adherence patterns in aortic dissection survivors. Indian J Med Res 2018;147:183-8. [Crossref] [PubMed]
- Martin G, Patel N, Grant Y, et al. Antihypertensive medication adherence in chronic type B aortic dissection is an important consideration in the management debate. J Vasc Surg 2018;68:693-699.e2. [Crossref] [PubMed]
Cite this article as: Arvin S, Ahmad K, Tang M, Andersen G, Nørgaard BL. Conservative treatment of type A aortic dissection: a case report with 5 years of follow-up. AME Case Rep 2023;7:42.


