
Surgical excision of a giant solitary keratoacanthoma in the cheek: a case report and literature review
Highlight box
Key findings
• Our report highlights the successful surgical excision of a giant keratoacanthoma, with no evidence of recurrence during the follow-up period. Intraoperative frozen-section examination can quickly evaluate the tumor margins and develop a surgical plan.
What is known and what is new?
• Keratoacanthomas are typically small, dome-shaped lesions that resemble squamous cell carcinomas (SCCs). Histopathological examination remains the reference standard for distinguishing SCC from keratoacanthoma.
• Giant keratoacanthomas may present a diagnostic challenge due to their atypical appearance. They can grow rapidly and become locally invasive. Intraoperative frozen-section examination played a crucial role in ensuring clear margins and complete tumor removal.
What is the implication, and what should change now?
• Clinicians should consider the possibility of giant keratoacanthomas in patients presenting with large, rapidly growing skin masses. Surgical excision with intraoperative frozen-section examination is an effective approach for the confirmation of diagnosis.
Introduction
Keratoacanthoma is a low-grade skin tumor characterized by a distinct subset of self-regressing, locally invasive and destructively expanding keratinocytic skin neoplasms with the micromorphology of squamous cell carcinoma (SCC) (1). Clinically, keratoacanthoma can be classified as solitary keratoacanthoma, multiple keratoacanthoma, eruptive keratoacanthoma (2) and keratoacanthoma centrifugum marginatum (3). Giant keratoacanthoma is a rare variant of solitary keratoacanthoma characterized by a diameter exceeding 20 mm, a self-limiting course, rapid enlargement within several weeks and spontaneous regression within several months (4). Complete surgical lesion removal is recommended for the treatment of solitary keratoacanthoma (5). Among reported cases, giant keratoacanthomas are very rare. We report a case of a giant keratoacanthoma of the cheek that was surgically excised and not observed to reoccur during follow-up. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-100/rc).
Case presentation
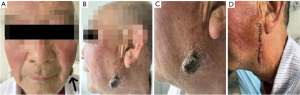
A 75-year-old male patient presented with a left facial mass that had persisted for more than 6 months. Throughout the aforementioned period, the patient experienced accelerated mass growth, itching, pain, swelling, purulent ulceration, and other discomforts. The patient had no family history of similar diseases or history of trauma preceding onset and had no obvious history of drug addiction or chemical exposure. At initial visit, the patient underwent history taking and physical examination, which revealed an exophytic mass on the left cheek with a transverse length of 25 mm and a longitudinal length of 30 mm located 30 mm below the earlobe (Figure 1). Cervical lymph nodes were not swollen. Based on the clinical history and physical examination, the preliminary consideration is “cheek mass, suspected to be giant keratoacanthoma”. To further confirm the diagnosis and proceed with treatment, the patient was admitted to Hospital of Stomatology, Tianjin Medical University on November 12, 2021 for further evaluation and treatment.
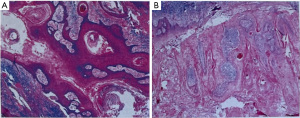
Surgery was performed under local anesthetic infiltration with 2% articaine. Meilan was used to mark the surgical incision within 5 mm of the tumor’s periphery. The lesion was completely removed from the left cheek, and approximately 5 mm from the edge of the lesion was visible to the naked eye. The skin flap was extended for esthetic purposes, and the flap was brought along the direction of skin striations for tension-reducing sutures to close the gap (Figure 2A-2E). The wound was subsequently closed with a 3-0 silk thread. After completely removing the neoplasm, the tissue was processed for intraoperative frozen section histopathological examination. The histopathological results are as follows: The surface of the keratoacanthoma is verrucous and cup-shaped architecture with a central keratin-filled crater; inflammatory infiltrate, including lymphocytes and neutrophils surrounding the tumor; focal mild epithelial dysplasia; absence of significant cellular atypia or invasion into the surrounding tissues (Figure 3A,3B).
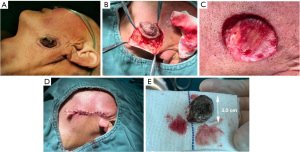
Postoperatively, the surgical site was scrubbed daily with iodophor for one week. No lesions recurred within the first postoperative week, and the wound healed properly. The lesion resolved completely, and the cheek function was normal (Figure 1D). The patient was followed up since the day of surgery, and his last follow-up was on July 17, 2023. There was no cheek discomfort during the treatment or follow-up periods. The patient had no obvious adverse reactions and had good prognosis, no recurrence, uncomplicated wound healing, and satisfactory cosmetic results. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Keratoacanthoma, also known historically as molluscum sebaceum, is a self-healing SCC. In the recent World Health Organization (WHO) classification of cutaneous tumors, keratoacanthoma was classified as a low-grade SCC, suggesting that it is biologically stable and that some keratoacanthomas evolve into conventional SCCs with a gradual loss of capacity for spontaneous regression (6). Individuals with keratoacanthoma are most likely to be men aged ≥60 years (7).
The histopathological stages of keratoacanthoma are as follows: early, proliferative, well-developed, regressing, and regressed (6). Based on distinctive clinical and histological features, keratoacanthomas can also be separated into follicular and non-follicular categories (8). Follicular lesions are located on the hair-bearing skin. Non-follicular lesions included subungual and mucous membrane keratoacanthomas. Solitary keratoacanthoma is a benign epithelial neoplasm with follicular differentiation. An increasing number of reports suggest that solitary keratoacanthomas can be located in the conjunctival, vulvar, and subungual regions (5). Some lesions occur in the external auditory canal, and a risk factor for this is the frequent use of earbuds (9). Careful monitoring after biopsy is advocated even if the histopathological diagnosis is keratoacanthoma because a conventional SCC may remain in the residual tissue (10).
The origin of keratoacanthoma has not been completely elucidated; however, causative factors include immunosuppression, ultraviolet (UV) or X-ray exposure, drug treatments, foreign bodies, various traumas (5), and human papillomavirus DNA. Male sex, older age, smoking, and alcohol consumption have been reported to be hazardous variables (7).
The true incidence of keratoacanthoma may be underestimated because of diagnosis as SCC, physician underreporting, or spontaneous regression before diagnosis. An essential clinical characteristic of solitary keratoacanthoma is its self-limiting course; however, not all keratoacanthomas resolve spontaneously (7). Currently, histopathological examination remains the reference standard for distinguishing SCC from keratoacanthoma. Histopathologically, keratoacanthoma is characterized by mature lesions surrounding an arched epithelium that forms a crater filled with keratin. An enlarged pale cytoplasm within cells is arranged in concentric-layered islands with central keratinization. These nests extend into the dermis to the level of sweat glands. Large cells with pink cytoplasm oriented towards the nest of central tumor cells are generally characteristic of keratoacanthoma (11).
In the literature concerning the use of dermoscopy and reflectance confocal microscopy in Chinese Han patients, the dermoscopic features of keratoacanthoma include concentric circles of the central crater, keratin mass, keratin scale, and polymorphic vascular pattern. Reflectance confocal microscopic features include refractile crust, atypical honeycomb pattern, dark-centered cells, large round nucleated cells, dendritic cells, and linear or round vessels traversing the dermal papillae (12). Currently, there is a web application in the latest research literature to process large numbers of free-text histopathology reports and classify diagnoses of keratinocytic cancers with a high degree of accuracy (13).
The treatment methods for keratoacanthoma can be divided into non-pharmacological, pharmacological, and surgical treatments (14,15). Surgical management is the standard treatment for solitary keratoacanthoma (5). Complete removal and follow-up are recommended for solitary keratoacanthoma within the proliferative phase. In the literature, treatment with intralesional methotrexate has been used as an important treatment for keratoacanthoma before surgical excision or as an adjuvant treatment for postoperative management (16). Intralesional methotrexate has produced a greater response in patient with keratoacanthoma than in those with SCC, indicating its effectiveness and safety (17).
Conclusions
The high risk of cosmetic problems should be considered after the surgical resection of a giant isolated keratoacanthoma on the face. Our elderly male patient had loose facial skin, an obvious Langer’s line, poor tension, and an incision of less than 40 mm. The skin flap was extended for esthetic purposes, and the flap was brought along the direction of skin striations for tension-reducing sutures to close the gap. When the invasion range of the tumor is large and a perforation defect is formed, free-flap transplantation should be performed for functional repair.
Acknowledgments
Funding: This work was supported by the Science and Technology Project of Tianjin Health Committee (Grant No. ZC20134).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-100/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-100/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-100/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nirenberg A, Steinman H, Dixon A. Keratoacanthoma: Update on the Debate. Am J Dermatopathol 2021;43:305-7. [Crossref] [PubMed]
- Vitiello M, Echeverria B, Romanelli P, et al. Multiple eruptive keratoacanthomas arising in a tattoo. J Clin Aesthet Dermatol 2010;3:54-5. [PubMed]
- Mehrtens SH, de la Hera I, Shankar S. Case of keratoacanthoma centrifugum marginatum treated with acitretin. BMJ Case Rep 2018;2018:bcr2018226818. [Crossref] [PubMed]
- Bogner PN, Cheney RT, Zeitouni NC. Giant keratoacanthoma: case report and review of the English literature. Am J Dermatopathol 2014;36:252-7. [Crossref] [PubMed]
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): An update and review. J Am Acad Dermatol 2016;74:1220-33. [Crossref] [PubMed]
- Ogita A, Ansai SI. What Is a Solitary Keratoacanthoma? A Benign Follicular Neoplasm, Frequently Associated with Squamous Cell Carcinoma. Diagnostics (Basel) 2021;11:1848. [Crossref] [PubMed]
- Kolmodin A, Pandeya NP, Olsen CM, et al. Patient and Tumour Characteristics of Keratoacanthoma in a Large, Community-based Cohort Study from Queensland, Australia. Acta Derm Venereol 2021;101:adv00469. [Crossref] [PubMed]
- Choonhakarn C, Ackerman AB. Keratoacanthomas: a new classification based on morphologic findings and on anatomic site. Dermatopathol Pract Concept 2001;7:7-16.
- Ash J, Limbu R, Alexander V, et al. Keratoacanthoma in the External Auditory Canal. Cureus 2021;13:e16873. [PubMed]
- Ansai SI, Umebayashi Y, Katsumata N, et al. Japanese Dermatological Association Guidelines: Outlines of Guidelines for Cutaneous Squamous Cell Carcinoma 2020. J Dermatol 2021;48:e288-311. [Crossref] [PubMed]
- Takai T. Advances in histopathological diagnosis of keratoacanthoma. J Dermatol 2017;44:304-14. [Crossref] [PubMed]
- Wang X, Wang Y, Wang H, et al. The first report of diagnosing of keratoacanthoma in Chinese Han patients using dermoscopy and reflectance confocal microscopy. Skin Res Technol 2021;27:422-7. [Crossref] [PubMed]
- Thompson BS, Hardy S, Pandeya N, et al. Web Application for the Automated Extraction of Diagnosis and Site From Pathology Reports for Keratinocyte Cancers. JCO Clin Cancer Inform 2020;4:711-23. [Crossref] [PubMed]
- Hoegler KM, Schleichert RA. Is the first-line treatment of keratoacanthomas surgical excision or injection of intralesional chemotherapy? J Am Acad Dermatol 2020;83:1542-3. [Crossref] [PubMed]
- Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The Effectiveness of Cryosurgery Combined with Curettage and Electrodessication in the Treatment of Keratoacanthoma: A Retrospective Analysis of 90 Cases. Indian J Dermatol 2020;65:406-8. [Crossref] [PubMed]
- Krema H, Gursel Ozkurt Z, Lando L, et al. Complete cure of a large resistant keratoacanthoma of the eyelid with intralesional methotrexate. Can J Ophthalmol 2021;56:e162-4. [Crossref] [PubMed]
- Gualdi G, Caravello S, Frasci F, et al. Intralesional Methotrexate for the Treatment of Advanced Keratinocytic Tumors: A Multi-Center Retrospective Study. Dermatol Ther (Heidelb) 2020;10:769-77. [Crossref] [PubMed]
Cite this article as: Ni Y, Li R, Zhu D, Dong R, Qiao F. Surgical excision of a giant solitary keratoacanthoma in the cheek: a case report and literature review. AME Case Rep 2024;8:7.


