
Choroidal metastasis of pancreatic adenocarcinoma: case report
Highlight box
Key findings
• Patient with stage 4 pancreatic adenocarcinoma found to have choroidal metastasis.
What is known and what is new?
• Choroidal metastasis of pancreatic adenocarcinoma is rare, with limited knowledge of response to chemotherapeutic regimens.
• We report a case of improvement in choroidal metastasis and patient-reported ocular symptoms with gemcitabine/paclitaxel-protein bound/cisplatin, and survival exceeding mean expectations.
What is the implication, and what should change now?
• While choroidal metastasis should not be missed in patients with pancreatic ductal adenocarcinoma, systemic chemotherapy may be effective in mitigating collateral symptomatology and thus preserving quality of life.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a solid tumor accounting for 90% of all pancreatic carcinomas (1). The five-year survival rate for pancreatic adenocarcinoma ranges from 2–9% worldwide, with a median survival of two to eleven months depending on tumor stage at detection. The lethality of this disease is primarily attributed to its typical late presentation, with metastasis or local advancement identified in 80–85% of patients at first diagnosis of PDAC (2).
Metastasis is most commonly seen in the liver, as well as the peritoneum, lung, and bones (3). Less common are metastasis to other sites such as the skin (4) and central nervous system (CNS) (5). Thus, a thorough evaluation is warranted in every case with PDAC including systemic imaging along with history and physical examination. We report a case of pancreatic adenocarcinoma with distant metastasis to the left choroid in accordance with the CARE reporting checklist (6) (available at https://acr.amegroups.com/article/view/10.21037/acr-23-86/rc).
Case presentation
Patient background
A 59-year-old Caucasian male initially presented to urgent care with right flank pain in early January 2022, at which time he was prescribed cyclobenzaprine for presumed musculoskeletal pain. Prior to this presentation, the patient had no significant medical history. The pain persisted despite this treatment and progressed to impair gym exercise as well as daily activity. The patient then presented to his primary care provider in February 2022. At this time, an abdominal computed tomography (CT) with contrast was ordered showing a pancreatic tail mass. Cancer antigen 19-9 (CA 19-9) was 448 U/mL. The patient subsequently underwent endoscopic ultrasound (EUS) with fine needle aspiration (FNA) confirming the pancreatic tail mass as PDAC. This was followed up with a positron emission tomography (PET) scan revealing a hypermetabolic focus within the pancreatic tail mass, as well as possible pleural and bone metastasis. This pleural metastasis was not confirmed due to high risk of biopsy. The bone metastasis was not biopsied either due to imaging confirmation and concern over yield of biopsy. Clinically, the patient was considered to be in stage 4 PDAC.
Germline testing through Ambry (Aliso Viejo, CA, USA) revealed pathogenic mutation in one copy of his cystic fibrosis transmembrane conductance regulator (CFTR) gene. FoundationOne CDx (Cambridge, MA, USA) testing of his peripheral blood revealed a KRAS G12D, FGFR4, GNAS, and TP53 pathogenic mutations. The patient did not have any barriers of access in pursuing appropriate workup and treatment.
In March 2022 systemic chemotherapy was initiated with gemcitabine/paclitaxel-protein bound/cisplatin. Prior to treatment administration, the patient reported acute onset of blurry vision in the left eye as well as intermittent headaches. The patient was referred to an ophthalmologist. Of note, a recent CT of the brain without contrast done on initial workup in February 2022 was unremarkable.
Choroidal metastasis
In April 2022, the patient presented to a retinal specialist for evaluation of his acute onset left sided blurry vision. The patient reported that images appeared to be smaller in his left central vision compared to his right, and that straight lines in the left eye appeared to be distorted. The patient endorsed intermittent episodes of “blue flashes” described as though “it was snowing outside” near the onset of the symptoms which had since ceased. The patient also endorsed intermittent headaches that he attributed to his right eye attempting to compensate for the left. He denied floaters, aggravating or alleviating factors, and impact on daily activities. Examination revealed left eye pigmentary changes overlying subretinal fluid (SRF) along with peripheral retinal depigmentation (Figure 1A-1C). The conclusion was the patient has evidence of metastatic disease to the left retina/choroid and not a central serous choroidopathy.
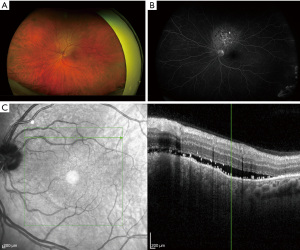
Therapeutic intervention
Beginning in March 2022, the patient initiated therapy with gemcitabine/paclitaxel-protein bound/cisplatin. Dosing followed prior published literature with gemcitabine given at 1,000 mg/m2, paclitaxel-protein bound at 125 mg/m2, and cisplatin at 25 mg/m2 (7). Patient tolerated therapy well other than minimal chemotherapy induced peripheral neuropathy affecting his fingers. He did undergo a partial splenic embolization procedure to improve thrombocytopenia secondary to hypersplenism. The patient did not have any tolerability issues outside of prior published literature that were unexpected (7). As of the submission of this report the patient is clinically doing well with a partial response to his initial chemotherapeutic regimen, including resolution of SRF at subsequent ophthalmologic visits (Figures 2,3). He remains active on his second line of chemotherapy.
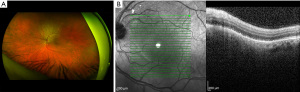
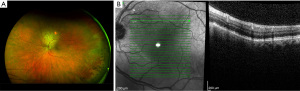
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Verbal informed consent for publication of this case report and accompanying images was obtained from the patient. Written informed consent was not obtained due to the patient and relatives being inaccessible at the time of submission of this report.
Discussion
We describe a case of PDAC with distant metastasis to the choroid. The patient is a 58-year-old male who initially complained of flank pain refractory to symptomatic treatment. A pancreatic tail mass was discovered on CT which was subsequently confirmed to be PDAC with FNA. At the time of diagnosis, the patient had evidence of metastasis to the pleura. Additionally, the patient began experiencing ocular symptoms including blurry vision, blue flashes, and headaches. Upon ophthalmological examination he was found to have pigmentary changes overlying SRF in the left eye indicating choroidal metastasis. The patient has since undergone systemic treatment with gemcitabine/abraxane/cisplatin. He has shown a partial response to therapy and is clinically doing well with some shrinkage of choroidal metastasis.
Choroidal metastasis is a rare manifestation of PDAC with limited prior documentation in literature. Previous studies describing characteristics of choroidal metastasis have found that pancreatic primary tumors account for less than 1–7% of cases (8-12). Shields et al. further found that PDAC primaries account for the lowest survival rates in cases of choroidal metastasis, with a mean survival of 4.2 months (13).
Our patient’s accumulation of SRF required careful distinguishing from central serous chorioretinopathy (CSC), a benign condition that typically affects males in the 2nd to 5th decade of life (14). A previous case report described misdiagnosis of CSC in a 45-year-old male that was subsequently found to be pancreatic metastasis (15). Another report described pancreatic choroidal metastasis appearing as cotton-wool spots in a 47-year-old male patient with no history of diabetes mellitus (16).
Though choroidal metastasis is a rare manifestation of PDAC, it is important to be aware of this gamut of presentations as it has previously acted as a herald of PDAC or its recurrence. In a 72-year-old woman, unilateral decrease in visual acuity was the first symptom leading to the discovery of advanced pancreatic carcinoma (17). A 59-year-old male who had undergone a Whipple procedure, radiation therapy, and chemotherapy for pancreatic adenocarcinoma presented four years later with decreased vision in the left eye, and was found to have extensive metastasis (18). Similarly, the aforementioned 45-year-old male patient initially diagnosed with CSC had a history of surgical resection for locally advanced pancreatic adenocarcinoma two years prior and was believed to be in remission (15).
Several treatment modalities may be used to treat choroidal metastasis of PDAC, including chemotherapy, plaque or external beam radiotherapy, and resection (19). Treatment of the primary tumor using gemcitabine/abraxane/cisplatin was successful in halting growth of choroidal lesions in this case. Similarly, treatment with gemcitabine/cisplatin in a 28-year-old male with PDAC resulted in total disappearance of ocular lesions (20). Local radiation resulted in regression of SRF in one case (15). A 62-year-old female PDAC patient who developed new cotton wool spots but did not have evidence of choroidal metastasis also saw shrinkage of these spots after Gemcitabine treatment, which may prompt investigation into non-anticancer effects of Gemcitabine on paraneoplastic retinopathies (16). Encouragingly, despite prior reports of a mean survival of 4.2 months, our patient remains active and is free of choroidal metastasis 11 months after his initial diagnosis of PDAC.
Conclusions
In this patient, the choroid was the first confirmed metastatic site and represented distant metastasis. Nevertheless, this patient continues to do well and is expected to exceed the upper bound median survival of 11 months following systemic chemotherapy. From this case, we note that distant metastasis prior to treatment initiation may not predict worse prognosis. In addition, systemic chemotherapy was effective in both primary tumor shrinkage as well as regression of choroidal metastasis, leading to improvement in visual symptoms. This suggests that while choroidal metastasis should not be missed in patients with PDAC, systemic chemotherapy may be effective in mitigating collateral symptomatology and thus preserving quality of life.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-86/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-86/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-86/coif). E.B. reports other financial or non-financial interests for consulting—BPG, Clearnote, Conjupro, Elevation Oncology, Nanology, Qurient, Vivacitus. R.S. is a private practitioner of Georgia Retina, a for-profit company. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Verbal informed consent for publication of this case report and accompanying images was obtained from the patient. Written informed consent was not obtained due to the patient and relatives being inaccessible at the time of submission of this report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Wang S, Zheng Y, Yang F, et al. The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct Target Ther 2021;6:249. [Crossref] [PubMed]
- Ito H, Tajiri T, Hiraiwa SI, et al. A Case of Rare Cutaneous Metastasis from Advanced Pancreatic Cancer. Case Rep Oncol 2020;13:49-54. [Crossref] [PubMed]
- Kumar A, Dagar M, Herman J, et al. CNS involvement in pancreatic adenocarcinoma: a report of eight cases from the Johns Hopkins Hospital and review of literature. J Gastrointest Cancer 2015;46:5-8. [Crossref] [PubMed]
- CARE checklist. Available online: https://www.care-statement.org/checklist. Published 2013. Accessed 2023
- Jameson GS, Borazanci E, Babiker HM, et al. Response Rate Following Albumin-Bound Paclitaxel Plus Gemcitabine Plus Cisplatin Treatment Among Patients With Advanced Pancreatic Cancer: A Phase 1b/2 Pilot Clinical Trial. JAMA Oncol 2020;6:125-32. [Crossref] [PubMed]
- Al-Dahmash SA, Shields CL, Kaliki S, et al. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina 2014;34:1588-93. [Crossref] [PubMed]
- Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol 2015;63:122-7. [Crossref] [PubMed]
- Shields CL, Kalafatis NE, Gad M, et al. Metastatic tumours to the eye. Review of metastasis to the iris, ciliary body, choroid, retina, optic disc, vitreous, and/or lens capsule. Eye (Lond) 2023;37:809-14. [Crossref] [PubMed]
- Wang TJ, Yang CM, Ho TC, et al. Metastatic choroidal tumors in Taiwan: an 11-year experience. Am J Ophthalmol 2005;140:735-7. [Crossref] [PubMed]
- Kang HG, Kim M, Byeon SH, et al. Clinical Spectrum of Uveal Metastasis in Korean Patients Based on Primary Tumor Origin. Ophthalmol Retina 2021;5:543-52. [Crossref] [PubMed]
- Shields CL, Welch RJ, Malik K, et al. Uveal Metastasis: Clinical Features and Survival Outcome of 2214 Tumors in 1111 Patients Based on Primary Tumor Origin. Middle East Afr J Ophthalmol 2018;25:81-90. [Crossref] [PubMed]
- Medina CA, Townsend JH, Singh AD, et al. Central serous chorioretinopathy. In: Manual of Retinal Diseases: A Guide to Diagnosis and Management. New York, NY: Springer; 2016:421-6.
- Smith E, Tran T. Recurrence of Pancreatic Cancer Presenting as Choroidal Metastasis: A Case Report. Case Rep Ophthalmol 2021;12:854-8. [Crossref] [PubMed]
- Schmidt D, Ness T, Geissler M. Cotton-wool spots associated with pancreatic carcinoma. Eur J Med Res 2001;6:101-4. [PubMed]
- Nasser M, Haj M, Nassar F. Carcinoma of pancreas presenting as a decrease in visual acuity. Hepatogastroenterology 2002;49:558-60. [PubMed]
- Hiniker A, Oakes SA, Rao RC. Bilateral Choroidal Metastases from Pancreatic Adenocarcinoma. Ophthalmology 2017;124:1825. [Crossref] [PubMed]
- Shah SU, Shields CL, Bianciotto CG, et al. Pancreatic cancer metastasis to choroid. Ophthalmology 2011;118:1483-1483.e4. [Crossref] [PubMed]
- Singh A, Malik D, Singh S, et al. Choroidal metastasis in pancreatic adenocarcinoma. J Cancer Res Ther 2022;18:263-5. [Crossref] [PubMed]
Cite this article as: Shriram J, Stoltz R, Borazanci E. Choroidal metastasis of pancreatic adenocarcinoma: case report. AME Case Rep 2024;8:22.


