
Thyroid storm triggered by thyroid nodule fine needle aspiration biopsy: a case report
Highlight box
Key findings
• We present a case of a 62-year-old female patient with history of multinodular goiter and Graves’ disease who experienced a thyroid storm instigated by fine needle aspiration (FNA) biopsy of the thyroid.
What is known and what is new?
• FNA is rarely needed in the case of a hyperfunctioning thyroid nodule, as it can be seen on radionuclide thyroid scan. Total thyroidectomy is warranted in a hyperthyroid state in an emergent setting without ample time for medical therapy to be effective.
• This case demonstrates that FNA biopsy and physical manipulation of the thyroid gland may precipitate a thyroid storm.
What is the implication, and what should change now?
• FNA biopsy of the thyroid gland should be used with caution in certain settings. When executed, a euthyroid state needs to be achieved before attempting to perform an FNA.
Introduction
Thyroid storm is an endocrinological emergency first described in 1926 (1). It is a potentially fatal manifestation of thyrotoxicosis with a mortality rate of approximately 10% that involves symptoms of hyperthyroidism and organ decompensation (1,2). Immediate treatment is necessary via medical/drug therapy, and if that fails, therapeutic plasma exchange or thyroidectomy is the next alternative (1). Survivors should be longitudinally medicated for underlying hyperthyroidism to prevent subsequent issues or recurrence (1).
Thyroid storm is most commonly caused by Graves’ disease, followed by toxic nodular goiter (3). Other triggers include iodine-induced and drug-induced thyroid dysfunction, thyroiditis, and ingestion of excess thyroid hormones (3). Although the literature on this topic is quite sparse and it is a rare occurrence, fine needle aspiration (FNA) biopsy in a patient with uncontrolled hyperthyroidism can instigate a thyroid storm. We present the case of a patient who had Graves’ disease and multinodular goiter (MNG) who had an FNA of a thyroid nodule, which precipitated a severe thyrotoxicosis requiring management in the intensive care unit (ICU). The patient ultimately underwent emergent thyroidectomy as the last resort to save her life. It is important to create awareness that complications like thyroid storm can happen after FNA thyroid biopsy as described in this case. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-63/rc).
Case presentation
A 62-year-old female with a past medical history of diabetes mellitus type 2, hypertension, obesity, deep vein thrombosis, and pulmonary embolism was diagnosed 3 years prior to admission with MNG and hyperthyroidism. Upon diagnosis 3 years priors, she was started on methimazole. She was not compliant with her medication, and less than a month before presenting to our institution, she was taking methimazole intermittently and switched to levothyroxine. There was no prior thyroid function test or obtainable medical records as she was diagnosed and treated in another country. The patient presented to the emergency department at our institution with complaints of palpitations and tachycardia. She reported a gradual increase in compression symptoms and voice changes for the entire prior month, of which the trigger was most likely her medication noncompliance. In the hospital, she was in mild distress with a dysphonic voice, tachypnea inability to speak in full sentences, tachycardia (heart rate of 120–130 beats per minute), and an extremely large goiter palpated with extension over the sternocleidomastoid bilaterally.
Upon initial evaluation, her thyroid stimulating hormone (TSH) was 0.05 mIU/mL (normal: 0.27–4.2 mIU/mL), free T4 was greater than 7.77 ng/dL (normal: 0.93–1.70 ng/dL), T3 was 552 ng/dL (normal: 80–200 ng/dL), and thyroid stimulating immunoglobulin (TSI) and TSH receptor antibody (TRAb) were elevated at 476% (normal: <130% basal activity) and 23 IU/L (normal: 0–0.9 IU/L), respectively. She underwent a chest computed tomography (CT) scan without intravenous iodine contrast injection which demonstrated a thyroid gland that was heterogeneous in appearance and markedly enlarged with a right thyroid lobe measuring approximately 6.2 cm × 5.8 cm and the left lobe measuring approximately 5.5 cm × 5.0 cm. There was resultant narrowing of the trachea measuring 6 mm in the transverse dimension at its narrowest point. Further evaluation with dedicated ultrasound of the thyroid showed bilateral MNG with coarse calcifications as well as a notable left thyroid cyst measuring 1.6 cm × 1.2 cm × 2.3 cm, isoechoic, with smooth margins. The thyroid nodule was classified as TIRADS 2 (based on score of 0 for cystic, 2 for hypoechoic, 0 for smooth, 0 for no artifacts, and 0 for wider-than tall). American Thyroid Association (ATA) classification was very low suspicion (purely cystic, no solid component; it could be argued that the nodule instead fit the ATA benign classification).
The patient was started on methimazole 40 mg daily, prednisone 20 mg daily, cholestyramine 4 mg four times a day, saturated solution of potassium iodine (SSKI) 50 mg three times per day, and propranolol for heart rate control. Another service recommended FNA biopsy of the right 3 cm thyroid nodule, which was done 2 days after she was admitted. Pathology demonstrated Bethesda II benign follicular nodular tissue.
One day after the FNA procedure, the patient developed altered mental status, stridor, and hypoxia with worsening tachycardia and hypertension. Her Burch-Wartofsky Point Scale score was about 55 points, which is highly suggestive of thyroid storm (4). She met the Japan Thyroid Association diagnostic criteria for grade TS1, exhibiting thyrotoxicosis and at least one central nervous system manifestation as well as fever, tachycardia, chronic heart failure, or gastrointestinal/hepatic manifestations (5). Her T3 was 135.9 ng/dL (normal: 80–200 ng/dL), T4 was 16.6 ng/dL (normal: 0.93–1.70 ng/dL), and TSH was 0.026 mclU/mL (normal: 0.27–4.2 mclU/mL). She was urgently transferred to the ICU and started on non-invasive ventilation per ICU team decision. She was also administered an increased steroid administration to stress dose. After a few hours of non-invasive ventilation, she was intubated to protect her airway. These newly onset symptoms were attributed to the FNA rather than to worsening of her respiratory status from another cause. Bleeding into the goiter and pulmonary embolism were ruled out via thyroid ultrasound and chest CT angiogram, respectively. Due to the large goiter compressing her neck, the ICU team could not pass the orogastric tube. Switching to oral propylthiouracil (PTU) was considered, but the patient could not receive PO medications, and thus, the decision was made to start PTU enema at 400 mg in normal saline every eight hours. Her free T4 decreased to 3.99 ng/dL and T3 to 165 ng/dL. Her clinical status remained unchanged, and she ultimately required ventilation and pressors while developing worsening transaminitis and leukocytosis.
At this point, her condition was poor, and she required further medical attention. A multidisciplinary meeting was held with the ICU, endocrine surgery, and endocrinology teams to discuss treatment options. A decision was made to proceed with total thyroidectomy despite the patient’s inability to achieve euthyroid status. This course of action was taken because the patient was clinically deteriorating, and the situation was emergent. Her procedure was well tolerated under aggressive beta-blockade without any subsequent clinical worsening. She was fortunately extubated the next day.
Her excised multinodular thyroid was described as mixed macro and microfollicular goiter with reactive fibrosis and degenerative changes, weighing 110 g. The right thyroid lobe measured 12 cm × 6 cm × 4 cm; the left was 12 cm × 6 cm × 3.5 cm, and the isthmus was 5 cm × 4 cm × 2 cm.
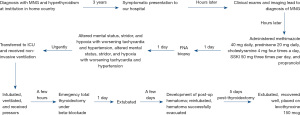
Her hospital course (Figure 1) following thyroidectomy was complicated. The patient initially clinically improved post-operatively and was taken off all antithyroid medications. However, she had to be reintubated a few days later due to airway compression from post-operative hematoma, which was evacuated. She was thereafter safely extubated again. She was placed on levothyroxine 150 mcg PO daily 5 days after surgery and did not develop postoperative hypocalcemia. She then recovered well, surviving this thyrotoxic experience.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying image. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Our patient had thyrotoxicosis on presentation secondary to Graves’ disease, which worsened to a thyroid storm after having an FNA of a thyroid nodule. It was unknown if she additionally had a hyperfunctioning thyroid nodule.
Thyroid storm is a severe, life-threatening manifestation of hyperthyroidism. Toxic MNG is the second leading cause of thyroid storm, just below Graves’ disease. Any primary cause of hyperthyroidism can turn into severe thyrotoxicosis. It may be precipitated by an acute event, such as surgery, acute iodine load, trauma, or thyroid manipulation. The specific underlying mechanism of the latter is unknown, but thought to be inflammation caused by biopsy, which triggers the release of thyroid hormone and increase in systemic thyroid hormone absorption via increased local vascularity. In the case of a cystic nodule, it has been proposed that the trigger could be attributed to leakage of thyroid content into the cyst, followed by release of thyroid hormone into the blood causing thyrotoxicosis (1).
Patients with known hyperthyroidism or low TSH with the presence of MNG should undergo uptake and scan before deciding on FNA thyroid biopsy. If the nodule is hyperfunctioning, FNA is unnecessary due to the low risk of malignancy. Occasionally, there may be nodules with high-risk features on ultrasound in which FNA may be considered. Some studies regarding Doppler flow in thyroid ultrasound posit that this modality can screen for hyperfunctioning thyroid nodules if a patient cannot undergo an uptake and scan (6,7). As per ATA guidelines, nodules should not be biopsied without knowing the patient’s thyroid activity level (8).
FNA is a simple, safe procedure that provides information as an initial screening for patients with thyroid nodules. Post-FNA local pain and minor hematomas are the most common complications. Post-aspiration thyrotoxicosis has been described in patients with a cystic component in the nodule that rarely presents with overt symptoms and demonstrates prompt resolution. Incidence of thyroiditis after post-aspiration is less than 1% (9); however, it is believed that post-aspiration thyrotoxicosis is underdiagnosed based on lack of overt thyrotoxic clinical symptoms (10). Post-FNA hyperthyroidism can also be caused by suppurative thyroiditis, which would require antibiotics and possibly drainage. Underlying diseases such as tuberculosis, diabetes, or immunosuppression increase the risk for developing a thyroid abscess. This risk is rare and related to improper cleaning technique (11).
Thyroidectomy should be avoided in hyperthyroid patients until a euthyroid state is achieved. Pre-treatment with methimazole is recommended to avoid severe thyrotoxicosis during surgery. In most cases, euthyroidism is achieved within six weeks of treatment. Potassium iodide has been used preoperatively to reduce the thyroid gland vascularity and reduce the thyroid hormone release. Beta-blockers can block the peripheral effects of thyroid hormone. Overall, propranolol and atenolol are the beta-blockers of choice when attempting thyroidectomy. It is important to note that the clearance of metoprolol and propranolol undergoes extensive hepatic metabolism, which is increased in hyperthyroidism (12). However, the oral clearance of renally excreted beta-blockers such as sotalol and atenolol are not altered in hyperthyroidism (12). It is unlikely that newer beta-blockers will exceed the effectiveness of propranolol in hyperthyroid thyroidectomy cases (12).
The treatment for a thyroid storm is similar for the uncomplicated hyperthyroidism, with medications given at higher doses and more frequently with additional drugs like glucocorticoids and cholestyramine, which decrease the conversion of T4 to T3 and enhances thyroid hormone clearance, respectively. A multidisciplinary team should be involved earlier if the patient is not responding to medications. It is important to have full support in the ICU. All measures should be tried before attempting surgery as emergent management.
Thyroidectomy can be performed in a dire, emergency setting when other options have been exhausted and there is no time for pharmacotherapy, as in our case. In addition, emergent surgical intervention can be done if the patient develops side effects from the treatment (severe thrombocytopenia or agranulocytosis) (1).
In 2016, Fischli et al. performed a study on ten patients with Graves’ disease and thyrotoxicosis who underwent thyroidectomy treated with beta-blockers, thionamides, and glucocorticoids at the time of surgery (13). Their study illustrated that fast and efficacious preoperative preparation of patients with hyperthyroidism is achievable with Lugol’s solution, dexamethasone, and a beta-blocker. These medications are beneficial at the time of thyroidectomy (13).
Older studies, such as the one performed by Lee et al. in 1982, have shown that the use of propranolol can decrease the duration of preparation of a hyperthyroid patient for thyroidectomy from weeks or months down to just 24 hours (14). An average daily dose of 160 mg of propranolol was used, with a range of 40 to 320 mg/day (14). There was no report of postoperative thyroid storm, nerve injury, or permanent hypoparathyroidism (14). There were also no changes in thyroid hormone levels intraoperatively, suggesting beta-blockers stop thyroid hormone release during thyroid gland manipulation. The antagonization of beta-receptor-mediated effects of systemic catecholamines (antiadrenergic) also inhibits excess thyroid hormone and prevents the peripheral conversion of T4 to T3 (15). Steroids that were started for thyrotoxicosis should be continued, and if not, stress dose steroids should be initiated prior to surgery.
Conclusions
FNA biopsy is a commonly used and generally safe procedure; however, patients with overt hyperthyroidism should not undergo FNA thyroid biopsy. Patients’ thyroid levels should be decreased prior to thyroid manipulation as to prevent a potential thyroid storm. Thyroidectomy should be avoided in hyperthyroid patients until a euthyroid state is achieved. However, it can be performed if necessary—if considered to be lifesaving in a setting without ample time for medical therapy to be effective, as seen in our patient. Beta-blockers, glucocorticoids, thionamides, and saturated solution of potassium iodine safely prevent thyroid storm and thyrotoxicosis before thyroidectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-63/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-63/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-63/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying image. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chiha M, Samarasinghe S, Kabaker AS. Thyroid storm: an updated review. J Intensive Care Med 2015;30:131-40. [Crossref] [PubMed]
- Angell TE, Lechner MG, Nguyen CT, et al. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab 2015;100:451-9. [Crossref] [PubMed]
- De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet 2016;388:906-18. [Crossref] [PubMed]
- De Groot LJ, Bartalena L, Feingold KR. Thyroid Storm. Figure 1. [Burch-Wartofsky Point Scale for the Diagnosis of Thyroid Storm. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext. South Dartmouth (MA): MDTextcom, Inc. 2022.
- Satoh T, Isozaki O, Suzuki A, et al. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr J 2016;63:1025-64.
- Chen L, Zhao X, Liu H, et al. Mean peak systolic velocity of the superior thyroid artery is correlated with radioactive iodine uptake in untreated thyrotoxicosis. J Int Med Res 2012;40:640-7. [Crossref] [PubMed]
- Donkol RH, Nada AM, Boughattas S. Role of color Doppler in differentiation of Graves' disease and thyroiditis in thyrotoxicosis. World J Radiol 2013;5:178-83. [Crossref] [PubMed]
- Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016;26:1343-421. [Crossref] [PubMed]
- Kobayashi A, Kuma K, Matsuzuka F, et al. Thyrotoxicosis after needle aspiration of thyroid cyst. J Clin Endocrinol Metab 1992;75:21-4. [PubMed]
- Onyekwelu E, Poyner T. Thyrotoxicosis following fine needle aspiration biopsy of a mixed thyroid mass: a case report and relevant review. Ann Thyroid 2021;6:5. [Crossref]
- Yildar M, Demirpolat G, Aydin M. Acute suppurative thyroiditis accompanied by thyrotoxicosis after fine-needle aspiration: treatment with catheter drainage. J Clin Diagn Res 2014;8:ND12-4. [Crossref] [PubMed]
- Feely J. Clinical pharmacokinetics of beta-adrenoceptor blocking drugs in thyroid disease. Clin Pharmacokinet 1983;8:1-16. [Crossref] [PubMed]
- Fischli S, Lucchini B, Müller W, et al. Rapid preoperative blockage of thyroid hormone production / secretion in patients with Graves' disease. Swiss Med Wkly 2016;146:w14243. [Crossref] [PubMed]
- Lee TC, Coffey RJ, Currier BM, et al. Propranolol and thyroidectomy in the treatment of thyrotoxicosis. Ann Surg 1982;195:766-73. [Crossref] [PubMed]
- Geffner DL, Hershman JM. Beta-adrenergic blockade for the treatment of hyperthyroidism. Am J Med 1992;93:61-8. [Crossref] [PubMed]
Cite this article as: Eatz TA, Rios P, Penaherrera Oviedo CA, Lagari V. Thyroid storm triggered by thyroid nodule fine needle aspiration biopsy: a case report. AME Case Rep 2024;8:15.


